Protective effector memory CD4 T cells depend on ICOS for survival
- PMID: 21364749
- PMCID: PMC3041765
- DOI: 10.1371/journal.pone.0016529
Protective effector memory CD4 T cells depend on ICOS for survival
Abstract
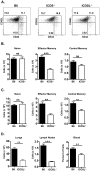
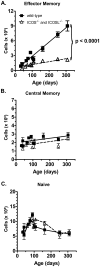
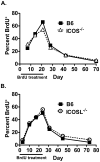
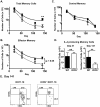
Memory CD4 T cells play a vital role in protection against re-infection by pathogens as diverse as helminthes or influenza viruses. Inducible costimulator (ICOS) is highly expressed on memory CD4 T cells and has been shown to augment proliferation and survival of activated CD4 T cells. However, the role of ICOS costimulation on the development and maintenance of memory CD4 T cells remains controversial. Herein, we describe a significant defect in the number of effector memory (EM) phenotype cells in ICOS(-/-) and ICOSL(-/-) mice that becomes progressively more dramatic as the mice age. This decrease was not due to a defect in the homeostatic proliferation of EM phenotype CD4 T cells in ICOS(-/-) or ICOSL(-/-) mice. To determine whether ICOS regulated the development or survival of EM CD4 T cells, we utilized an adoptive transfer model. We found no defect in development of EM CD4 T cells, but long-term survival of ICOS(-/-) EM CD4 T cells was significantly compromised compared to wild-type cells. The defect in survival was specific to EM cells as the central memory (CM) ICOS(-/-) CD4 T cells persisted as well as wild type cells. To determine the physiological consequences of a specific defect in EM CD4 T cells, wild-type and ICOS(-/-) mice were infected with influenza virus. ICOS(-/-) mice developed significantly fewer influenza-specific EM CD4 T cells and were more susceptible to re-infection than wild-type mice. Collectively, our findings demonstrate a role for ICOS costimulation in the maintenance of EM but not CM CD4 T cells.
Conflict of interest statement
Figures

Similar articles
-
Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4+ T cells.J Immunol. 2000 Nov 1;165(9):5035-40. doi: 10.4049/jimmunol.165.9.5035. J Immunol. 2000. PMID: 11046032
-
ICOS/ICOSL interaction is required for CD4+ invariant NKT cell function and homeostatic survival.J Immunol. 2008 Apr 15;180(8):5448-56. doi: 10.4049/jimmunol.180.8.5448. J Immunol. 2008. PMID: 18390727 Free PMC article.
-
Opposing effects of ICOS on graft-versus-host disease mediated by CD4 and CD8 T cells.J Immunol. 2006 Jun 15;176(12):7394-401. doi: 10.4049/jimmunol.176.12.7394. J Immunol. 2006. PMID: 16751384
-
Regulation of CD4 T cell activation and effector function by inducible costimulator (ICOS).Curr Opin Immunol. 2010 Jun;22(3):326-32. doi: 10.1016/j.coi.2010.01.001. Epub 2010 Jan 29. Curr Opin Immunol. 2010. PMID: 20116985 Review.
-
The role of ICOS in allergic disease: Positive or Negative?Int Immunopharmacol. 2022 Feb;103:108394. doi: 10.1016/j.intimp.2021.108394. Epub 2021 Dec 16. Int Immunopharmacol. 2022. PMID: 34922247 Review.
Cited by
-
CD3+ICOS+ T cells show differences in the synthesis of nitric oxide, IFN-γ, and IL-10 in patients with pulmonary tuberculosis or in healthy household contacts.Clin Exp Med. 2016 Nov;16(4):481-491. doi: 10.1007/s10238-015-0380-3. Epub 2015 Aug 8. Clin Exp Med. 2016. PMID: 26253701
-
Loss of Immune Tolerance Is Controlled by ICOS in Sle1 Mice.J Immunol. 2016 Jul 15;197(2):491-503. doi: 10.4049/jimmunol.1502241. Epub 2016 Jun 13. J Immunol. 2016. PMID: 27296665 Free PMC article.
-
'Nur'turing tumor T cell tolerance and exhaustion: novel function for Nuclear Receptor Nur77 in immunity.Eur J Immunol. 2020 Nov;50(11):1643-1652. doi: 10.1002/eji.202048869. Epub 2020 Oct 25. Eur J Immunol. 2020. PMID: 33063848 Free PMC article. Review.
-
Cutting Edge: ICOS-Deficient Regulatory T Cells Display Normal Induction of Il10 but Readily Downregulate Expression of Foxp3.J Immunol. 2019 Feb 15;202(4):1039-1044. doi: 10.4049/jimmunol.1801266. Epub 2019 Jan 14. J Immunol. 2019. PMID: 30642977 Free PMC article.
-
A combination trial of vaccine plus ipilimumab in metastatic castration-resistant prostate cancer patients: immune correlates.Cancer Immunol Immunother. 2014 Apr;63(4):407-18. doi: 10.1007/s00262-014-1524-0. Epub 2014 Feb 11. Cancer Immunol Immunother. 2014. PMID: 24514956 Free PMC article. Clinical Trial.
References
-
- Swain SL, Dutton RW, Woodland DL. T cell responses to influenza virus infection: effector and memory cells. Viral Immunol. 2004;17:197–209. - PubMed
-
- Xu R, Johnson AJ, Liggitt D, Bevan MJ. Cellular and humoral immunity against vaccinia virus infection of mice. J Immunol. 2004;172:6265–6271. - PubMed
-
- Palmer DR, Krzych U. Cellular and molecular requirements for the recall of IL-4-producing memory CD4(+)CD45RO(+)CD27(-) T cells during protection induced by attenuated Plasmodium falciparum sporozoites. Eur J Immunol. 2002;32:652–661. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

