A gene expression signature of acquired chemoresistance to cisplatin and fluorouracil combination chemotherapy in gastric cancer patients
- PMID: 21364753
- PMCID: PMC3041770
- DOI: 10.1371/journal.pone.0016694
A gene expression signature of acquired chemoresistance to cisplatin and fluorouracil combination chemotherapy in gastric cancer patients
Abstract
Background: We initiated a prospective trial to identify transcriptional alterations associated with acquired chemotherapy resistance from pre- and post-biopsy samples from the same patient and uncover potential molecular pathways involved in treatment failure to help guide therapeutic alternatives.
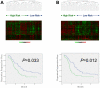
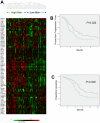
Methodology/principal findings: A prospective, high-throughput transcriptional profiling study was performed using endoscopic biopsy samples from 123 metastatic gastric cancer patients prior to cisplatin and fluorouracil (CF) combination chemotherapy. 22 patients who initially responded to CF were re-biopsied after they developed resistance to CF. An acquired chemotherapy resistance signature was identified by analyzing the gene expression profiles from the matched pre- and post-CF treated samples. The acquired resistance signature was able to segregate a separate cohort of 101 newly-diagnosed gastric cancer patients according to the time to progression after CF. Hierarchical clustering using a 633-gene acquired resistance signature (feature selection at P<0.01) separated the 101 pretreatment patient samples into two groups with significantly different times to progression (2.5 vs. 4.7 months). This 633-gene signature included the upregulation of AKT1, EIF4B, and RPS6 (mTOR pathway), DNA repair and drug metabolism genes, and was enriched for genes overexpressed in embryonic stem cell signatures. A 72-gene acquired resistance signature (a subset of the 633 gene signature also identified in ES cell-related gene sets) was an independent predictor for time to progression (adjusted P = 0.011) and survival (adjusted P = 0.034) of these 101 patients.
Conclusion/significance: This signature may offer new insights into identifying new targets and therapies required to overcome the acquired resistance of gastric cancer to CF.
Conflict of interest statement
Figures

References
-
- Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5(4):275–284. - PubMed
-
- Hess KR, Anderson K, Symmans WF, Valero V, Ibrahim N, et al. Pharmacogenomic predictor of sensitivity to preoperative chemotherapy with paclitaxel and fluorouracil, doxorubicin, and cyclophosphamide in breast cancer. J Clin Oncol. 2006;24(26):4236–4244. - PubMed
-
- Kang Y, Kang WK, Shin DB, Chen J, Xiong J, et al. Randomized phase III trial of capecitabine/cisplatin vs continuous infusion of 5-FU/cisplatin as first-line therapy in patients with advanced gastric cancer: efficacy and safety results. 18sJ Clin Oncol. 2006;24 (suppl; abstr 4018)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

