Gliadin-mediated proliferation and innate immune activation in celiac disease are due to alterations in vesicular trafficking
- PMID: 21364874
- PMCID: PMC3045409
- DOI: 10.1371/journal.pone.0017039
Gliadin-mediated proliferation and innate immune activation in celiac disease are due to alterations in vesicular trafficking
Abstract
Background and objectives: Damage to intestinal mucosa in celiac disease (CD) is mediated both by inflammation due to adaptive and innate immune responses, with IL-15 as a major mediator of the innate immune response, and by proliferation of crypt enterocytes as an early alteration of CD mucosa causing crypts hyperplasia. We have previously shown that gliadin peptide P31-43 induces proliferation of cell lines and celiac enterocytes by delaying degradation of the active epidermal growth factor receptor (EGFR) due to delayed maturation of endocytic vesicles. IL-15 is increased in the intestine of patients affected by CD and has pleiotropic activity that ultimately results in immunoregulatory cross-talk between cells belonging to the innate and adaptive branches of the immune response. Aims of this study were to investigate the role of P31-43 in the induction of cellular proliferation and innate immune activation.
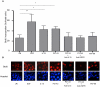
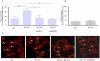
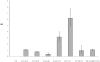
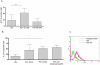
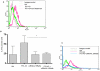
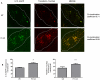
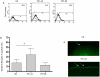
Methods/principal findings: Cell proliferation was evaluated by bromodeoxyuridine (BrdU) incorporation both in CaCo-2 cells and in biopsies from active CD cases and controls. We used real-time PCR to evaluate IL-15 mRNA levels and FACS as well as ELISA and Western Blot (WB) analysis to measure protein levels and distribution in CaCo-2 cells. Gliadin and P31-43 induce a proliferation of both CaCo-2 cells and CD crypt enterocytes that is dependent on both EGFR and IL-15 activity. In CaCo-2 cells, P31-43 increased IL-15 levels on the cell surface by altering intracellular trafficking. The increased IL-15 protein was bound to IL15 receptor (IL-15R) alpha, did not require new protein synthesis and functioned as a growth factor.
Conclusion: In this study, we have shown that P31-43 induces both increase of the trans-presented IL-15/IL5R alpha complex on cell surfaces by altering the trafficking of the vesicular compartments as well as proliferation of crypt enterocytes with consequent remodelling of CD mucosa due to a cooperation of IL-15 and EGFR.
Conflict of interest statement
Figures
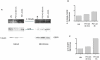


References
-
- Sollid LM. Molecular basis of celiac disease. Annu Rev Immunol. 2000;2000;18:53–81. - PubMed
-
- Maiuri L, Ciacci C, Ricciardelli I, Vacca L, Raia V, et al. Association between innate response to gliadin and activation of pathogenic T cells in coeliac disease. Lancet. 2003;362:30–37. - PubMed
-
- Hue S, Mention JJ, Monteiro RC, Zhang S, Cellier C, et al. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity. 2004;21:367–77. - PubMed
-
- Marsh MN, Crowe PT. Morphology of the mucosal lesion in gluten sensitivity. Baillieres Clin Gastroenterol. 1995;9:273–93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

