Toward the defined and xeno-free differentiation of functional human pluripotent stem cell-derived retinal pigment epithelial cells
- PMID: 21364903
- PMCID: PMC3044694
Toward the defined and xeno-free differentiation of functional human pluripotent stem cell-derived retinal pigment epithelial cells
Abstract
Purpose: The production of functional retinal pigment epithelium (RPE) cells from human embryonic (hESCs) and human induced pluripotent stem cells (hiPSCs) in defined and xeno-free conditions is highly desirable, especially for their use in cell therapy for retinal diseases. In addition, differentiated RPE cells provide an individualized disease model and drug discovery tool. In this study, we report the differentiation of functional RPE-like cells from several hESC lines and one hiPSC line in culture conditions, enabling easy translation to clinical quality cell production under Good Manufacturing Practice regulations.
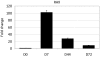
Methods: Pluripotent stem cells were cultured on human fibroblast feeder cells in serum-free medium. The differentiation toward RPE was induced by removing basic fibroblast growth factor and feeder cells from the serum-free conditions. RPE differentiation was also achieved using xeno-free and defined culture conditions. The RPE cell morphology and pigmentation of the cells were analyzed and the expression of genes and proteins characteristic for RPE cells was evaluated. In vitro functionality of the cells was analyzed using ELISA measurements for pigment epithelium derived factor (PEDF) secretion and phagocytosis of photoreceptor outer segments (POS). The integrity of the generated RPE layers was analyzed using transepithelial electric resistance measurements.
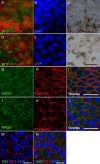
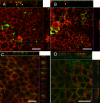
Results: We generated putative RPE cells with typical pigmented cobblestone-like morphology. The expression of RPE-specific markers was confirmed at the gene and protein level. The differentiated cells were able to phagocytose POS and secrete PEDF characteristic of native RPE cells. In addition, cultured cells formed a polarized epithelium with high integrity and exhibited excellent transepithelial electric resistance values, indicating well established, tight junctions. Moreover, we introduced an improved method to generate functional putative RPE cells without xeno-components under defined conditions.
Conclusions: We have developed a progressive differentiation protocol for the production of functional RPE-like cells from hESCs and hiPSCs. Our results demonstrate that putative hESC-RPE and hiPSC-RPE express genes and proteins characteristic for RPE cells, as well as being able to phagocytose POS and secrete PEDF. Furthermore, our results show that RPE-like cells can be differentiated in xeno-free and defined culture conditions, which is mandatory for Good Manufacturing Practice-production of these cells for clinical use.
Figures


Similar articles
-
Xeno- and feeder-free differentiation of human pluripotent stem cells to two distinct ocular epithelial cell types using simple modifications of one method.Stem Cell Res Ther. 2017 Dec 29;8(1):291. doi: 10.1186/s13287-017-0738-4. Stem Cell Res Ther. 2017. PMID: 29284513 Free PMC article.
-
Aquaporin expression and function in human pluripotent stem cell-derived retinal pigmented epithelial cells.Invest Ophthalmol Vis Sci. 2013 May 1;54(5):3510-9. doi: 10.1167/iovs.13-11800. Invest Ophthalmol Vis Sci. 2013. PMID: 23687169
-
Defined Medium Conditions for the Induction and Expansion of Human Pluripotent Stem Cell-Derived Retinal Pigment Epithelium.Stem Cell Rev Rep. 2016 Apr;12(2):179-88. doi: 10.1007/s12015-015-9636-2. Stem Cell Rev Rep. 2016. PMID: 26589197
-
Pluripotent Stem Cells to Model Degenerative Retinal Diseases: The RPE Perspective.Adv Exp Med Biol. 2019;1186:1-31. doi: 10.1007/978-3-030-28471-8_1. Adv Exp Med Biol. 2019. PMID: 31654384 Review.
-
Production of human pluripotent stem cell therapeutics under defined xeno-free conditions: progress and challenges.Stem Cell Rev Rep. 2015 Feb;11(1):96-109. doi: 10.1007/s12015-014-9544-x. Stem Cell Rev Rep. 2015. PMID: 25077810 Free PMC article. Review.
Cited by
-
Efflux protein expression in human stem cell-derived retinal pigment epithelial cells.PLoS One. 2012;7(1):e30089. doi: 10.1371/journal.pone.0030089. Epub 2012 Jan 17. PLoS One. 2012. PMID: 22272278 Free PMC article.
-
A Step by Step Protocol for Subretinal Surgery in Rabbits.J Vis Exp. 2016 Sep 13;(115):53927. doi: 10.3791/53927. J Vis Exp. 2016. PMID: 27684952 Free PMC article.
-
Pigment Epithelia of the Eye: Cell-Type Conversion in Regeneration and Disease.Life (Basel). 2022 Mar 6;12(3):382. doi: 10.3390/life12030382. Life (Basel). 2022. PMID: 35330132 Free PMC article. Review.
-
Fabricating retinal pigment epithelial cell sheets derived from human induced pluripotent stem cells in an automated closed culture system for regenerative medicine.PLoS One. 2019 Mar 13;14(3):e0212369. doi: 10.1371/journal.pone.0212369. eCollection 2019. PLoS One. 2019. PMID: 30865653 Free PMC article.
-
Texture Descriptors Ensembles Enable Image-Based Classification of Maturation of Human Stem Cell-Derived Retinal Pigmented Epithelium.PLoS One. 2016 Feb 19;11(2):e0149399. doi: 10.1371/journal.pone.0149399. eCollection 2016. PLoS One. 2016. PMID: 26895509 Free PMC article.
References
-
- Klimanskaya I, Hipp J, Rezai KA, West M, Atala A, Lanza R. Derivation and comparative assessment of retinal pigment epithelium from human embryonic stem cells using transcriptomics. Cloning Stem Cells. 2004;6:217–45. - PubMed
-
- Enseleit F, Michels S, Ruschitzka F. Anti-VEGF therapies and blood pressure: more than meets the eye. Curr Hypertens Rep. 2010;12:33–8. - PubMed
-
- Coffey PJ, Girman S, Wang SM, Hetherington L, Keegan DJ, Adamson P, Greenwood J, Lund RD. Long-term preservation of cortically dependent visual function in RCS rats by transplantation. Nat Neurosci. 2002;5:53–6. - PubMed
-
- da Cruz L, Chen FK, Ahmado A, Greenwood J, Coffey P. RPE transplantation and its role in retinal disease. Prog Retin Eye Res. 2007;26:598–635. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
