O-methylguanine-DNA methyltransferase (MGMT) mRNA expression predicts outcome in malignant glioma independent of MGMT promoter methylation
- PMID: 21365007
- PMCID: PMC3041820
- DOI: 10.1371/journal.pone.0017156
O-methylguanine-DNA methyltransferase (MGMT) mRNA expression predicts outcome in malignant glioma independent of MGMT promoter methylation
Abstract
Background: We analyzed prospectively whether MGMT (O(6)-methylguanine-DNA methyltransferase) mRNA expression gains prognostic/predictive impact independent of MGMT promoter methylation in malignant glioma patients undergoing radiotherapy with concomitant and adjuvant temozolomide or temozolomide alone. As DNA-methyltransferases (DNMTs) are the enzymes responsible for setting up and maintaining DNA methylation patterns in eukaryotic cells, we analyzed further, whether MGMT promoter methylation is associated with upregulation of DNMT expression.
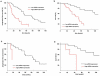
Methodology/principal findings: ADULT PATIENTS WITH A HISTOLOGICALLY PROVEN MALIGNANT ASTROCYTOMA (GLIOBLASTOMA: N = 53, anaplastic astrocytoma: N = 10) were included. MGMT promoter methylation was determined by methylation-specific PCR (MSP) and sequencing analysis. Expression of MGMT and DNMTs mRNA were analysed by real-time qPCR. Prognostic factors were obtained from proportional hazards models. Correlation between MGMT mRNA expression and MGMT methylation status was validated using data from the Cancer Genome Atlas (TCGA) database (N = 229 glioblastomas). Low MGMT mRNA expression was strongly predictive for prolonged time to progression, treatment response, and length of survival in univariate and multivariate models (p<0.0001); the degree of MGMT mRNA expression was highly correlated with the MGMT promoter methylation status (p<0.0001); however, discordant findings were seen in 12 glioblastoma patients: Patients with methylated tumors with high MGMT mRNA expression (N = 6) did significantly worse than those with low transcriptional activity (p<0.01). Conversely, unmethylated tumors with low MGMT mRNA expression (N = 6) did better than their counterparts. A nearly identical frequency of concordant and discordant findings was obtained by analyzing the TCGA database (p<0.0001). Expression of DNMT1 and DNMT3b was strongly upregulated in tumor tissue, but not correlated with MGMT promoter methylation and MGMT mRNA expression.
Conclusions/significance: MGMT mRNA expression plays a direct role for mediating tumor sensitivity to alkylating agents. Discordant findings indicate methylation-independent pathways of MGMT expression regulation. DNMT1 and DNMT3b are likely to be involved in CGI methylation. However, their exact role yet has to be defined.
Conflict of interest statement
Figures



References
-
- Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005;64:479–489. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. 352/10/987 [pii] ;10.1056/NEJMoa043330 [doi] - DOI - PubMed
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. S1470-2045(09)70025-7 [pii] ; 10.1016/S1470-2045(09)70025-7 [doi] - DOI - PubMed
-
- Hegi ME, Diserens AC, Gorlia T, Hamou MF, de TN, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. 352/10/997 [pii] ; 10.1056/NEJMoa043331 [doi] - DOI - PubMed
-
- Wick W, Hartmann C, Engel C, Stoffels M, Felsberg J, et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27:5874–5880. JCO.2009.23.6497 [pii]; 10.1200/JCO.2009.23.6497 [doi] - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

