Structural mechanism associated with domain opening in gain-of-function mutations in SHP2 phosphatase
- PMID: 21365683
- PMCID: PMC3076527
- DOI: 10.1002/prot.22984
Structural mechanism associated with domain opening in gain-of-function mutations in SHP2 phosphatase
Abstract
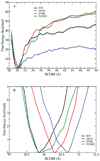
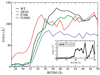
The SHP2 phosphatase plays a central role in a number of signaling pathways were it dephosphorylates various substrate proteins. Regulation of SHP2 activity is, in part, achieved by an intramolecular interaction between the PTP domain of the protein, which contains the catalytic site, and the N-SH2 domain leading to a "closed" protein conformation and autoinhibition. Accordingly, "opening" of the N-SH2 and PTP domains is required for the protein to become active. Binding of phosphopeptides to the N-SH2 domain is known to induce the opening event, while a number of gain-of-function (GOF) mutants, implicated in Noonan's Syndrome and childhood leukemias, are thought to facilitate opening. In the present study, a combination of computational and experimental methods are used to investigate the structural mechanism of opening of SHP2 and the impact of three GOF mutants, D61G, E76K, and N308D, on the opening mechanism. Calculated free energies of opening indicate that opening must be facilitated by effector molecules, possibly the protein substrates themselves, as the calculated free energies preclude spontaneous opening. Simulations of both wild type (WT) SHP2 and GOF mutants in the closed state indicate GOF activity to involve increased solvent exposure of selected residues, most notably Arg362, which in turn may enhance interactions of SHP2 with its substrate proteins and thereby aid opening. In addition, GOF mutations cause structural changes in the phosphopeptide-binding region of the N-SH2 domain leading to conformations that mimic the bound state. Such conformational changes are suggested to enhance binding of phosphopeptides and/or decrease interactions between the PTP and N-SH2 domains thereby facilitating opening. Experimental assays of the impact of effector molecules on SHP2 phosphatase activity against both small molecule and peptide substrates support the hypothesized mechanism of GOF mutant action. The present calculations also suggest a role for the C-SH2 domain of SHP2 in stabilizing the overall conformation of the protein in the open state, thereby aiding conformational switching between the open active and closed inactive states.
Copyright © 2011 Wiley-Liss, Inc.
Figures





References
-
- Xiao S, Rose DW, Sasaoka T, Maegawa H, Burke TR, Jr, Roller PP, Shoelson SE, Olefsky JM. Syp (SH-PTP2) is a positive mediator of growth factor-stimulated mitogenic signal transduction. J Biol Chem. 1994;269(33):21244–21248. - PubMed
-
- Rivard N, McKenzie FR, Brondello JM, Pouyssegur J. The phosphotyrosine phosphatase PTP1D, but not PTP1C, is an essential mediator of fibroblast proliferation induced by tyrosine kinase and G protein-coupled receptors. J Biol Chem. 1995;270(18):11017–11024. - PubMed
-
- Yu M, Luo J, Yang W, Wang Y, Mizuki M, Kanakura Y, Besmer P, Neel BG, Gu H. The scaffolding adapter Gab2, via Shp-2, regulates kit-evoked mast cell proliferation by activating the Rac/JNK pathway. J Biol Chem. 2006;281(39):28615–28626. - PubMed
-
- Neel BG, Gu H, Pao L. The 'Shp'ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28(6):284–293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

