Multiple roles of component proteins in bacterial multicomponent monooxygenases: phenol hydroxylase and toluene/o-xylene monooxygenase from Pseudomonas sp. OX1
- PMID: 21366224
- PMCID: PMC3059347
- DOI: 10.1021/bi200028z
Multiple roles of component proteins in bacterial multicomponent monooxygenases: phenol hydroxylase and toluene/o-xylene monooxygenase from Pseudomonas sp. OX1
Abstract
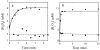
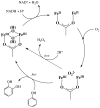
Phenol hydroxylase (PH) and toluene/o-xylene monooxygenase (ToMO) from Pseudomonas sp. OX1 require three or four protein components to activate dioxygen for the oxidation of aromatic substrates at a carboxylate-bridged diiron center. In this study, we investigated the influence of the hydroxylases, regulatory proteins, and electron-transfer components of these systems on substrate (phenol; NADH) consumption and product (catechol; H(2)O(2)) generation. Single-turnover experiments revealed that only complete systems containing all three or four protein components are capable of oxidizing phenol, a major substrate for both enzymes. Under ideal conditions, the hydroxylated product yield was ∼50% of the diiron centers for both systems, suggesting that these enzymes operate by half-sites reactivity mechanisms. Single-turnover studies indicated that the PH and ToMO electron-transfer components exert regulatory effects on substrate oxidation processes taking place at the hydroxylase actives sites, most likely through allostery. Steady state NADH consumption assays showed that the regulatory proteins facilitate the electron-transfer step in the hydrocarbon oxidation cycle in the absence of phenol. Under these conditions, electron consumption is coupled to H(2)O(2) formation in a hydroxylase-dependent manner. Mechanistic implications of these results are discussed.
Figures



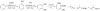

References
-
- Murray LJ, Lippard SJ. Substrate Trafficking and Dioxygen Activation in Bacterial Multicomponent Monooxygenases. Acc. Chem. Res. 2007;40:466–474. - PubMed
-
- Sazinsky MH, Lippard SJ. Correlating Structure with Function in Bacterial Multicomponent Monooxygenases and Related Diiron Proteins. Acc. Chem. Res. 2006;39:558–566. - PubMed
-
- Leahy JG, Batchelor PJ, Morcomb SM. Evolution of the Soluble Diiron Monooxygenases. FEMS Microbiol. Rev. 2003;27:449–479. - PubMed
-
- Notomista E, Lahm A, Di Donato A, Tramontano A. Evolution of Bacterial and Archaeal Multicomponent Monooxygenases. J. Mol. Evol. 2003;56:435–445. - PubMed
-
- Wallar BJ, Lipscomb JD. Dioxygen Activation by Enzymes Containing Binuclear Non-Heme Iron Clusters. Chem. Rev. 1996;96:2625–2657. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

