Sex differences in the rapid control of aromatase activity in the quail preoptic area
- PMID: 21366731
- PMCID: PMC3075373
- DOI: 10.1111/j.1365-2826.2011.02121.x
Sex differences in the rapid control of aromatase activity in the quail preoptic area
Abstract
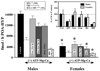
Adult male quail show high levels of aromatase activity in the preoptic area-hypothalamus (POA-HYP), which parallels the high number of aromatase-immunoreactive cells and elevated mRNA concentrations detected in this brain region by in situ hybridisation. Interestingly, females display considerably lower aromatase activity than males but have almost equal numbers of aromatase-immunoreactive cells and express similar levels of aromatase mRNA. Aromatase activity in the male POA-HYP can be rapidly regulated by calcium-dependent phosphorylations, in the absence of changes in enzyme concentration. In the present study, we investigated whether aromatase activity is differentially regulated by phosphorylations in males and females. A linear increase in accumulation of aromatisation products was observed in both sexes as a function of time but the rate of conversion was slower in females. Saturation analysis confirmed the lower maximum velocities (V(max) ) in females but indicated a similar affinity (K(m) ) in both sexes. Aromatase activity in females reacted differentially to manipulations of intracellular calcium. In particular, chelating calcium with ethylene glycol tetraacetic acid (EGTA) resulted in a larger increase of enzymatic activity in males than in females, especially in the presence of ATP. A differential reaction to kinase inhibitors was also observed between males and females (i.e. a larger increase in aromatase activity in females than in males after exposure to specific inhibitors). These findings suggest that the nature of aromatase is conserved between the sexes, although the control of its activity by calcium appears to be different. Additional characterizations of intracellular calcium in both sexes would therefore be appropriate to better understand aromatase regulation.
© 2011 The Authors. Journal of Neuroendocrinology © 2011 Blackwell Publishing Ltd.
Figures




References
-
- Balthazart J. Steroid metabolism and the activation of social behavior. In: Balthazart J, editor. Advances in Comparative and Environmental Physiology, vol 3. 1 ed. Berlin: Springer Verlag; 1989. pp. 105–159.
-
- Lephart ED. A review of brain aromatase cytochrome P450. Brain Res Rev. 1996;22:1–26. - PubMed
-
- Boon WC, Chow JD, Simpson ER. The multiple roles of estrogens and the enzyme aromatase. Prog Brain Res. 2010;181:209–232. - PubMed
-
- Balthazart J, Baillien M, Cornil CA, Ball GF. Preoptic aromatase modulates male sexual behavior: slow and fast mechanisms of action. Physiol Behav. 2004;83:247–270. - PubMed
-
- Adkins EK, Adler NT. Hormonal control of behavior in the Japanese quail. J Comp Physiol Psychol. 1972;81:27–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

