Treatment of a human papillomavirus type 31b-positive cell line with benzo[a]pyrene increases viral titer through activation of the Erk1/2 signaling pathway
- PMID: 21367897
- PMCID: PMC3126175
- DOI: 10.1128/JVI.00133-11
Treatment of a human papillomavirus type 31b-positive cell line with benzo[a]pyrene increases viral titer through activation of the Erk1/2 signaling pathway
Abstract
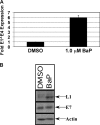
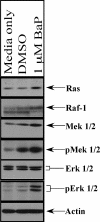
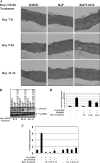
Numerous epidemiological studies have implicated cigarette smoking as a cofactor in the progression to cervical cancer. Tobacco-associated hydrocarbons have been found in cervical mucus, suggesting a possible interaction with human papillomavirus (HPV)-infected cells. The polycyclic aromatic hydrocarbon benzo[a]pyrene (BaP) is a major component of cigarette smoke condensate that has received significant attention due to its ability to induce carcinogenesis. We have previously demonstrated by conventional methods for determining viral titer that high concentrations of BaP increase HPV31b titers within the context of organotypic raft cultures compared with the level for vehicle controls. However, a definitive mechanism for explaining this increase in viral titer was lacking. Here, we show that BaP treatment activates the Ras-Raf-Mek1/2-Erk1/2 signaling pathway. The importance of Erk1/2 pathway activation to the BaP-mediated increase in viral titer was determined by Erk pathway inhibition with multiple Erk1/2 pathway inhibitors. Finally, BaP treatment activated p90RSK and its downstream target CDK1. These data indicate that the Erk1/2 signaling pathway plays an important role in mediating the response to BaP treatment that ultimately leads to increased viral titers.
Figures



Similar articles
-
Downregulation of Cdc2/CDK1 kinase activity induces the synthesis of noninfectious human papillomavirus type 31b virions in organotypic tissues exposed to benzo[a]pyrene.J Virol. 2010 May;84(9):4630-45. doi: 10.1128/JVI.02431-09. Epub 2010 Feb 24. J Virol. 2010. PMID: 20181698 Free PMC article.
-
[Benzo (a) pyrene-induced human embryo lung cell cycle alterations through positive regulation of mitogen-activated protein kinase signal pathways].Zhonghua Yu Fang Yi Xue Za Zhi. 2007 Jul;41(4):277-80. Zhonghua Yu Fang Yi Xue Za Zhi. 2007. PMID: 17959047 Chinese.
-
The cigarette smoke carcinogen benzo[a]pyrene enhances human papillomavirus synthesis.J Virol. 2008 Jan;82(2):1053-8. doi: 10.1128/JVI.01813-07. Epub 2007 Nov 7. J Virol. 2008. PMID: 17989183 Free PMC article.
-
Benzo[a]pyrene, but not 2,3,7,8-TCDD, induces G2/M cell cycle arrest, p21CIP1 and p53 phosphorylation in human choriocarcinoma JEG-3 cells: a distinct signaling pathway.Placenta. 2005 Apr;26 Suppl A:S87-95. doi: 10.1016/j.placenta.2005.01.013. Placenta. 2005. PMID: 15837074
-
High-Risk Human Papillomavirus and Tobacco Smoke Interactions in Epithelial Carcinogenesis.Cancers (Basel). 2020 Aug 6;12(8):2201. doi: 10.3390/cancers12082201. Cancers (Basel). 2020. PMID: 32781676 Free PMC article. Review.
Cited by
-
Human Papillomavirus and Cellular Pathways: Hits and Targets.Pathogens. 2021 Feb 25;10(3):262. doi: 10.3390/pathogens10030262. Pathogens. 2021. PMID: 33668730 Free PMC article. Review.
-
Onco-Pathogen Mediated Cancer Progression and Associated Signaling Pathways in Cancer Development.Pathogens. 2023 May 28;12(6):770. doi: 10.3390/pathogens12060770. Pathogens. 2023. PMID: 37375460 Free PMC article. Review.
-
The role of inflammation in HPV infection of the Oesophagus.BMC Cancer. 2013 Apr 9;13:185. doi: 10.1186/1471-2407-13-185. BMC Cancer. 2013. PMID: 23570247 Free PMC article.
-
Molecular mechanisms of viral oncogenesis in humans.Nat Rev Microbiol. 2018 Nov;16(11):684-698. doi: 10.1038/s41579-018-0064-6. Nat Rev Microbiol. 2018. PMID: 30143749 Free PMC article. Review.
-
Heparanase, cell signaling, and viral infections.Cell Mol Life Sci. 2020 Dec;77(24):5059-5077. doi: 10.1007/s00018-020-03559-y. Epub 2020 May 27. Cell Mol Life Sci. 2020. PMID: 32462405 Free PMC article. Review.
References
-
- Appleby P., et al. 2006. Carcinoma of the cervix and tobacco smoking: collaborative reanalysis of individual data on 13,541 women with carcinoma of the cervix and 23,017 women without carcinoma of the cervix from 23 epidemiological studies. Int. J. Cancer 118:1481–1495 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

