Regulation of antinucleoprotein IgG by systemic vaccination and its effect on influenza virus clearance
- PMID: 21367900
- PMCID: PMC3126167
- DOI: 10.1128/JVI.00150-11
Regulation of antinucleoprotein IgG by systemic vaccination and its effect on influenza virus clearance
Abstract
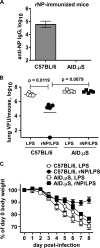
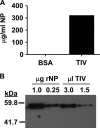
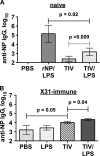
Seasonal influenza epidemics recur due to antigenic drift of envelope glycoprotein antigens and immune evasion of circulating viruses. Additionally, antigenic shift can lead to influenza pandemics. Thus, a universal vaccine that protects against multiple influenza virus strains could alleviate the continuing impact of this virus on human health. In mice, accelerated clearance of a new viral strain (cross-protection) can be elicited by prior infection (heterosubtypic immunity) or by immunization with the highly conserved internal nucleoprotein (NP). Both heterosubtypic immunity and NP-immune protection require antibody production. Here, we show that systemic immunization with NP readily accelerated clearance of a 2009 pandemic H1N1 influenza virus isolate in an antibody-dependent manner. However, human immunization with trivalent inactivated influenza virus vaccine (TIV) only rarely and modestly boosted existing levels of anti-NP IgG. Similar results were observed in mice, although the reaction could be enhanced with adjuvants, by adjusting the stoichiometry among NP and other vaccine components, and by increasing the interval between TIV prime and boost. Importantly, mouse heterosubtypic immunity that had waned over several months could be enhanced by injecting purified anti-NP IgG or by boosting with NP protein, correlating with a long-lived increase in anti-NP antibody titers. Thus, current immunization strategies poorly induce NP-immune antibody that is nonetheless capable of contributing to long-lived cross-protection. The high conservation of NP antigen and the known longevity of antibody responses suggest that the antiviral activity of anti-NP IgG may provide a critically needed component of a universal influenza vaccine.
Figures

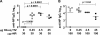

Similar articles
-
Contributions of antinucleoprotein IgG to heterosubtypic immunity against influenza virus.J Immunol. 2011 Apr 1;186(7):4331-9. doi: 10.4049/jimmunol.1003057. Epub 2011 Feb 25. J Immunol. 2011. PMID: 21357542 Free PMC article.
-
Headless hemagglutinin-containing influenza viral particles direct immune responses toward more conserved epitopes.J Virol. 2024 Oct 22;98(10):e0116624. doi: 10.1128/jvi.01166-24. Epub 2024 Sep 26. J Virol. 2024. PMID: 39324791 Free PMC article.
-
Greater Breadth of Vaccine-Induced Immunity in Females than Males Is Mediated by Increased Antibody Diversity in Germinal Center B Cells.mBio. 2022 Aug 30;13(4):e0183922. doi: 10.1128/mbio.01839-22. Epub 2022 Jul 20. mBio. 2022. PMID: 35856618 Free PMC article.
-
Impacts on influenza A(H1N1)pdm09 infection from cross-protection of seasonal trivalent influenza vaccines and A(H1N1)pdm09 vaccines: systematic review and meta-analyses.Vaccine. 2012 May 2;30(21):3209-22. doi: 10.1016/j.vaccine.2012.02.048. Epub 2012 Mar 2. Vaccine. 2012. PMID: 22387221
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
Cited by
-
Intranasal Immunization with Pressure Inactivated Avian Influenza Elicits Cellular and Humoral Responses in Mice.PLoS One. 2015 Jun 9;10(6):e0128785. doi: 10.1371/journal.pone.0128785. eCollection 2015. PLoS One. 2015. PMID: 26056825 Free PMC article.
-
Vaccination potential of B and T epitope-enriched NP and M2 against Influenza A viruses from different clades and hosts.PLoS One. 2018 Jan 29;13(1):e0191574. doi: 10.1371/journal.pone.0191574. eCollection 2018. PLoS One. 2018. PMID: 29377916 Free PMC article.
-
Immunity to the conserved influenza nucleoprotein reduces susceptibility to secondary bacterial infections.J Immunol. 2012 Nov 15;189(10):4921-9. doi: 10.4049/jimmunol.1201916. Epub 2012 Oct 1. J Immunol. 2012. PMID: 23028058 Free PMC article.
-
PapMV nanoparticles improve mucosal immune responses to the trivalent inactivated flu vaccine.J Nanobiotechnology. 2014 May 3;12:19. doi: 10.1186/1477-3155-12-19. J Nanobiotechnology. 2014. PMID: 24885884 Free PMC article.
-
Cellular senescence is a double-edged sword in regulating aged immune responses to influenza.Aging Cell. 2024 Jul;23(7):e14162. doi: 10.1111/acel.14162. Epub 2024 Apr 30. Aging Cell. 2024. PMID: 38689516 Free PMC article.
References
-
- Amanna I. J., Carlson N. E., Slifka M. K. 2007. Duration of humoral immunity to common viral and vaccine antigens. N. Engl. J. Med. 357:1903–1915 - PubMed
-
- Bodewes R., et al. 2010. Vaccination with whole inactivated virus vaccine affects the induction of heterosubtypic immunity against influenza virus A/H5N1 and immunodominance of virus-specific CD8+ T-cell responses in mice. J. Gen. Virol. 91:1743–1753 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 AI079537/AI/NIAID NIH HHS/United States
- R24 AO054953/AO/NIAID NIH HHS/United States
- R01 AI072689/AI/NIAID NIH HHS/United States
- K23 AI67501-01A1/AI/NIAID NIH HHS/United States
- AI61511/AI/NIAID NIH HHS/United States
- N01 AI050020/AI/NIAID NIH HHS/United States
- R01 AI061511/AI/NIAID NIH HHS/United States
- R24 AI054953/AI/NIAID NIH HHS/United States
- AI72689/AI/NIAID NIH HHS/United States
- 2662005500029C/PHS HHS/United States
- R01 AI068056/AI/NIAID NIH HHS/United States
- N01-AI50029/AI/NIAID NIH HHS/United States
- U54 CA260563/CA/NCI NIH HHS/United States
- N01 AI050029/AI/NIAID NIH HHS/United States
- AI079537/AI/NIAID NIH HHS/United States
- K23 AI067501/AI/NIAID NIH HHS/United States
- AI68056-05S1/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

