Axonally derived neuregulin-1 is required for remyelination and regeneration after nerve injury in adulthood
- PMID: 21368034
- PMCID: PMC3059576
- DOI: 10.1523/JNEUROSCI.2568-10.2011
Axonally derived neuregulin-1 is required for remyelination and regeneration after nerve injury in adulthood
Abstract
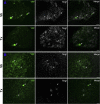
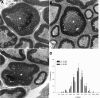
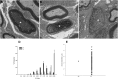
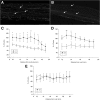
Neuregulin-1 (NRG1) plays a crucial role in axoglial signaling during the development of the peripheral nervous system, but its importance in adulthood after peripheral nerve injury remains unclear. We used single-neuron labeling with inducible Cre-mediated knock-out animals, which enabled visualization of a subset of adult myelinated sensory and motoneurons neurons in which Nrg1 was inducibly mutated by tamoxifen treatment. In uninjured mice, NRG1-deficient axons and the associated myelin sheath were normal, and the neuromuscular junction demonstrated normal apposition of presynaptic and postsynaptic components. After sciatic nerve crush, NRG1 ablation resulted in severe defects in remyelination: axons were either hypomyelinated or had no myelin sheath. NRG1-deficient axons were also found to regenerate at a slower rate. After nerve injury, the neuromuscular junction was reinnervated, but excess terminal sprouting was observed. Juxtacrine Neuregulin-1 signaling is therefore dispensable for maintenance of the myelin sheath in adult animals but has a key role in reparative processes after nerve injury.
Figures



References
-
- Bermingham-McDonogh O, Xu YT, Marchionni MA, Scherer SS. Neuregulin expression in PNS neurons: isoforms and regulation by target interactions. Mol Cell Neurosci. 1997;10:184–195. - PubMed
-
- Brinkmann BG, Agarwal A, Sereda MW, Garratt AN, Müller T, Wende H, Stassart RM, Nawaz S, Humml C, Velanac V, Radyushkin K, Goebbels S, Fischer TM, Franklin RJ, Lai C, Ehrenreich H, Birchmeier C, Schwab MH, Nave KA. Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron. 2008;59:581–595. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
