Complement protein C1q-mediated neuroprotection is correlated with regulation of neuronal gene and microRNA expression
- PMID: 21368058
- PMCID: PMC3080046
- DOI: 10.1523/JNEUROSCI.3932-10.2011
Complement protein C1q-mediated neuroprotection is correlated with regulation of neuronal gene and microRNA expression
Abstract
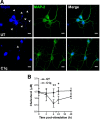
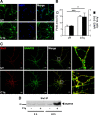
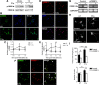
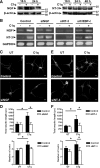
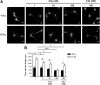
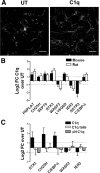
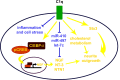
Activation of the complement cascade, a powerful effector mechanism of the innate immune system, is associated with neuroinflammation but also with elimination of inappropriate synapses during development. Synthesis of C1q, a recognition component of the complement system, occurs in brain during ischemia/reperfusion and Alzheimer's disease, suggesting that C1q may be a response to injury. In vitro, C1q, in the absence of other complement proteins, improves neuronal viability and neurite outgrowth and prevents β-amyloid-induced neuronal death, suggesting that C1q may have a direct neuroprotective role. Here, investigating the molecular basis for this neuroprotection in vitro, addition of C1q to rat primary cortical neurons significantly upregulated expression of genes associated with cholesterol metabolism, such as cholesterol-25-hydroxylase and insulin induced gene 2, and transiently decreased cholesterol levels in neurons, known to facilitate neurite outgrowth. In addition, the expression of syntaxin-3 and its functional association with synaptosomal-associated protein 25 was increased. C1q also increased the nuclear translocation of cAMP response element-binding protein and CCAAT/enhancer-binding protein-δ (C/EBP-δ), two transcription factors involved in nerve growth factor (NGF) expression and downregulated specific microRNAs, including let-7c that is predicted to target (and thus inhibit) NGF and neurotrophin-3 (NT-3) mRNA. Accordingly, C1q increased expression of NGF and NT-3, and small interfering RNA inhibition of C/EBP-δ, NGF, or NT-3 expression prevented the C1q-dependent neurite outgrowth. No such neuroprotective effect is seen in the presence of C3a or C5a. Finally, the induced neuronal gene expression required conformationally intact C1q. These results show that C1q can directly promote neuronal survival, thereby demonstrating new interactions between immune proteins and neuronal cells that may facilitate neuroprotection.
Figures

References
-
- Bénard M, Raoult E, Vaudry D, Leprince J, Falluel-Morel A, Gonzalez BJ, Galas L, Vaudry H, Fontaine M. Role of complement anaphylatoxin receptors (C3aR, C5aR) in the development of the rat cerebellum. Mol Immunol. 2008;45:3767–3774. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
