Sequenced response of extracellular matrix deadhesion and fibrotic regulators after muscle damage is involved in protection against future injury in human skeletal muscle
- PMID: 21368102
- PMCID: PMC3101036
- DOI: 10.1096/fj.10-176487
Sequenced response of extracellular matrix deadhesion and fibrotic regulators after muscle damage is involved in protection against future injury in human skeletal muscle
Abstract
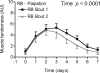
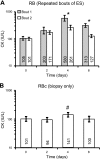
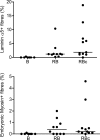
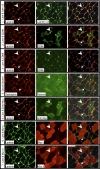
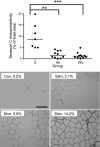
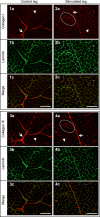
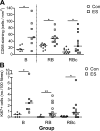
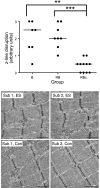
The purpose of this study was to test the hypothesis that remodeling of skeletal muscle extracellular matrix (ECM) is involved in protecting human muscle against injury. Biopsies were obtained from medial gastrocnemius muscles after a single bout of electrical stimulation (B) or a repeated bout (RB) 30 d later, or 30 d after a single stimulation bout (RBc). A muscle biopsy was collected from the control leg for comparison with the stimulated leg. Satellite cell content, tenascin C, and muscle regeneration were assessed by immunohistochemistry; real-time PCR was used to measure mRNA levels of collagens, laminins, heat-shock proteins (HSPs), inflammation, and related growth factors. The large responses of HSPs, CCL2, and tenascin C detected 48 h after a single bout were attenuated in the RB trial, indicative of protection against injury. Satellite cell content and 12 target genes, including IGF-1, were elevated 30 d after a single bout. Among those displaying the greatest difference vs. control muscle, ECM laminin-β1 and collagen types I and III were elevated ∼6- to 9-fold (P<0.001). The findings indicate that the sequenced events of load-induced early deadhesion and later strengthening of skeletal muscle ECM play a role in protecting human muscle against future injury.
Figures


Similar articles
-
Extracellular matrix remodeling and its contribution to protective adaptation following lengthening contractions in human muscle.FASEB J. 2015 Jul;29(7):2894-904. doi: 10.1096/fj.14-266668. Epub 2015 Mar 25. FASEB J. 2015. PMID: 25808538
-
Activation of satellite cells and the regeneration of human skeletal muscle are expedited by ingestion of nonsteroidal anti-inflammatory medication.FASEB J. 2016 Jun;30(6):2266-81. doi: 10.1096/fj.201500198R. Epub 2016 Mar 2. FASEB J. 2016. PMID: 26936358 Free PMC article. Clinical Trial.
-
Alcohol Differentially Alters Extracellular Matrix and Adhesion Molecule Expression in Skeletal Muscle and Heart.Alcohol Clin Exp Res. 2015 Aug;39(8):1330-40. doi: 10.1111/acer.12771. Epub 2015 Jun 24. Alcohol Clin Exp Res. 2015. PMID: 26108259 Free PMC article.
-
Skeletal muscle fibrosis: an overview.Cell Tissue Res. 2019 Mar;375(3):575-588. doi: 10.1007/s00441-018-2955-2. Epub 2018 Nov 12. Cell Tissue Res. 2019. PMID: 30421315 Review.
-
Alignment, cross linking, and beyond: a collagen architect's guide to the skeletal muscle extracellular matrix.Am J Physiol Cell Physiol. 2023 Oct 1;325(4):C1017-C1030. doi: 10.1152/ajpcell.00287.2023. Epub 2023 Sep 4. Am J Physiol Cell Physiol. 2023. PMID: 37661921 Free PMC article. Review.
Cited by
-
Skeletal muscle fibrosis is associated with decreased muscle inflammation and weakness in patients with chronic kidney disease.Am J Physiol Renal Physiol. 2018 Dec 1;315(6):F1658-F1669. doi: 10.1152/ajprenal.00314.2018. Epub 2018 Oct 3. Am J Physiol Renal Physiol. 2018. PMID: 30280599 Free PMC article.
-
The Structure and Role of Intramuscular Connective Tissue in Muscle Function.Front Physiol. 2020 May 19;11:495. doi: 10.3389/fphys.2020.00495. eCollection 2020. Front Physiol. 2020. PMID: 32508678 Free PMC article. Review.
-
The molecular basis for load-induced skeletal muscle hypertrophy.Calcif Tissue Int. 2015 Mar;96(3):196-210. doi: 10.1007/s00223-014-9925-9. Epub 2014 Oct 31. Calcif Tissue Int. 2015. PMID: 25359125 Free PMC article. Review.
-
The breaking and making of healthy adult human skeletal muscle in vivo.Skelet Muscle. 2017 Nov 7;7(1):24. doi: 10.1186/s13395-017-0142-x. Skelet Muscle. 2017. PMID: 29115986 Free PMC article.
-
Control of satellite cell function in muscle regeneration and its disruption in ageing.Nat Rev Mol Cell Biol. 2022 Mar;23(3):204-226. doi: 10.1038/s41580-021-00421-2. Epub 2021 Oct 18. Nat Rev Mol Cell Biol. 2022. PMID: 34663964 Review.
References
-
- Round J. M., Jones D. A., Cambridge G. (1987) Cellular infiltrates in human skeletal muscle: exercise induced damage as a model for inflammatory muscle disease? J. Neurol. Sci. 82, 1–11 - PubMed
-
- Hikida R. S., Staron R. S., Hagerman F. C., Sherman W. M., Costill D. L. (1983) Muscle fiber necrosis associated with human marathon runners. J. Neurol. Sci. 59, 185–203 - PubMed
-
- Mackey A. L., Bojsen-Moller J., Qvortrup K., Langberg H., Suetta C., Kalliokoski K. K., Kjaer M., Magnusson S. P. (2008) Evidence of skeletal muscle damage following electrically stimulated isometric muscle contractions in humans. J. Appl. Physiol. 105, 1620–1627 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

