Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice
- PMID: 21368114
- PMCID: PMC3053975
- DOI: 10.1073/pnas.1019581108
Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice
Expression of concern in
-
Editorial Expression of Concern: Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice.Proc Natl Acad Sci U S A. 2018 Jun 19;115(25):E5834. doi: 10.1073/pnas.1808586115. Epub 2018 Jun 11. Proc Natl Acad Sci U S A. 2018. PMID: 29891666 Free PMC article. No abstract available.
Abstract
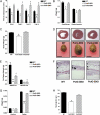
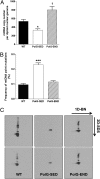
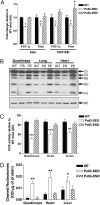
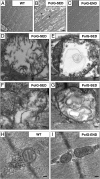
A causal role for mitochondrial DNA (mtDNA) mutagenesis in mammalian aging is supported by recent studies demonstrating that the mtDNA mutator mouse, harboring a defect in the proofreading-exonuclease activity of mitochondrial polymerase gamma, exhibits accelerated aging phenotypes characteristic of human aging, systemic mitochondrial dysfunction, multisystem pathology, and reduced lifespan. Epidemiologic studies in humans have demonstrated that endurance training reduces the risk of chronic diseases and extends life expectancy. Whether endurance exercise can attenuate the cumulative systemic decline observed in aging remains elusive. Here we show that 5 mo of endurance exercise induced systemic mitochondrial biogenesis, prevented mtDNA depletion and mutations, increased mitochondrial oxidative capacity and respiratory chain assembly, restored mitochondrial morphology, and blunted pathological levels of apoptosis in multiple tissues of mtDNA mutator mice. These adaptations conferred complete phenotypic protection, reduced multisystem pathology, and prevented premature mortality in these mice. The systemic mitochondrial rejuvenation through endurance exercise promises to be an effective therapeutic approach to mitigating mitochondrial dysfunction in aging and related comorbidities.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Exercise-induced mitochondrial p53 repairs mtDNA mutations in mutator mice.Skelet Muscle. 2016 Jan 31;6:7. doi: 10.1186/s13395-016-0075-9. eCollection 2016. Skelet Muscle. 2016. Retraction in: Skelet Muscle. 2021 Mar 30;11(1):8. doi: 10.1186/s13395-021-00264-7. PMID: 26834962 Free PMC article. Retracted.
-
Defects in mitochondrial DNA replication and oxidative damage in muscle of mtDNA mutator mice.Free Radic Biol Med. 2014 Oct;75:241-51. doi: 10.1016/j.freeradbiomed.2014.07.038. Epub 2014 Aug 12. Free Radic Biol Med. 2014. PMID: 25106705
-
Amelioration of premature aging in mtDNA mutator mouse by exercise: the interplay of oxidative stress, PGC-1α, p53, and DNA damage. A hypothesis.Curr Opin Genet Dev. 2016 Jun;38:127-132. doi: 10.1016/j.gde.2016.06.011. Epub 2016 Aug 3. Curr Opin Genet Dev. 2016. PMID: 27497229 Free PMC article. Review.
-
The mtDNA mutator mouse: Dissecting mitochondrial involvement in aging.Aging (Albany NY). 2009 Dec 11;1(12):1028-32. doi: 10.18632/aging.100109. Aging (Albany NY). 2009. PMID: 20157586 Free PMC article.
-
Oxidative stress, mitochondria and mtDNA-mutator mice.Exp Gerontol. 2006 Dec;41(12):1220-2. doi: 10.1016/j.exger.2006.10.018. Epub 2006 Nov 28. Exp Gerontol. 2006. PMID: 17126516 Free PMC article. Review.
Cited by
-
Increased mitochondrial biogenesis in muscle improves aging phenotypes in the mtDNA mutator mouse.Hum Mol Genet. 2012 May 15;21(10):2288-97. doi: 10.1093/hmg/dds049. Epub 2012 Feb 21. Hum Mol Genet. 2012. PMID: 22357654 Free PMC article.
-
Transient systemic mtDNA damage leads to muscle wasting by reducing the satellite cell pool.Hum Mol Genet. 2013 Oct 1;22(19):3976-86. doi: 10.1093/hmg/ddt251. Epub 2013 Jun 10. Hum Mol Genet. 2013. PMID: 23760083 Free PMC article.
-
The role of PGC-1 coactivators in aging skeletal muscle and heart.IUBMB Life. 2012 Mar;64(3):231-41. doi: 10.1002/iub.608. Epub 2012 Jan 25. IUBMB Life. 2012. PMID: 22279035 Free PMC article. Review.
-
Mito-SiPE is a sequence-independent and PCR-free mtDNA enrichment method for accurate ultra-deep mitochondrial sequencing.Commun Biol. 2022 Nov 19;5(1):1269. doi: 10.1038/s42003-022-04182-2. Commun Biol. 2022. PMID: 36402890 Free PMC article.
-
Quality control systems in cardiac aging.Ageing Res Rev. 2015 Sep;23(Pt A):101-15. doi: 10.1016/j.arr.2015.02.003. Epub 2015 Feb 19. Ageing Res Rev. 2015. PMID: 25702865 Free PMC article. Review.
References
-
- Harman D. The biologic clock: The mitochondria? J Am Geriatr Soc. 1972;20:145–147. - PubMed
-
- Larsson NG. Somatic mitochondrial DNA mutations in mammalian aging. Annu Rev Biochem. 2010;79:683–706. - PubMed
-
- Corral-Debrinski M, et al. Mitochondrial DNA deletions in human brain: Regional variability and increase with advanced age. Nat Genet. 1992;2:324–329. - PubMed
-
- Schwarze SR, et al. High levels of mitochondrial DNA deletions in skeletal muscle of old rhesus monkeys. Mech Ageing Dev. 1995;83:91–101. - PubMed
-
- Khaidakov M, Heflich RH, Manjanatha MG, Myers MB, Aidoo A. Accumulation of point mutations in mitochondrial DNA of aging mice. Mutat Res. 2003;526:1–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

