Rewiring hydrogenase-dependent redox circuits in cyanobacteria
- PMID: 21368150
- PMCID: PMC3053959
- DOI: 10.1073/pnas.1016026108
Rewiring hydrogenase-dependent redox circuits in cyanobacteria
Abstract
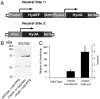
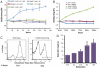
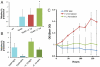
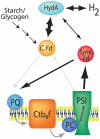
Hydrogenases catalyze the reversible reaction 2H(+) + 2e(-) ↔ H(2) with an equilibrium constant that is dependent on the reducing potential of electrons carried by their redox partner. To examine the possibility of increasing the photobiological production of hydrogen within cyanobacterial cultures, we expressed the [FeFe] hydrogenase, HydA, from Clostridium acetobutylicum in the non-nitrogen-fixing cyanobacterium Synechococcus elongatus sp. 7942. We demonstrate that the heterologously expressed hydrogenase is functional in vitro and in vivo, and that the in vivo hydrogenase activity is connected to the light-dependent reactions of the electron transport chain. Under anoxic conditions, HydA activity is capable of supporting light-dependent hydrogen evolution at a rate > 500-fold greater than that supported by the endogenous [NiFe] hydrogenase. Furthermore, HydA can support limited growth solely using H(2) and light as the source of reducing equivalents under conditions where Photosystem II is inactivated. Finally, we demonstrate that the addition of exogenous ferredoxins can modulate redox flux in the hydrogenase-expressing strain, allowing for greater hydrogen yields and for dark fermentation of internal energy stores into hydrogen gas.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Evolutionary significance of an algal gene encoding an [FeFe]-hydrogenase with F-domain homology and hydrogenase activity in Chlorella variabilis NC64A.Planta. 2011 Oct;234(4):829-43. doi: 10.1007/s00425-011-1431-y. Epub 2011 Jun 5. Planta. 2011. PMID: 21643991
-
[FeFe]- and [NiFe]-hydrogenase diversity, mechanism, and maturation.Biochim Biophys Acta. 2015 Jun;1853(6):1350-69. doi: 10.1016/j.bbamcr.2014.11.021. Epub 2014 Nov 24. Biochim Biophys Acta. 2015. PMID: 25461840 Review.
-
Proton Transfer Mechanisms in Bimetallic Hydrogenases.Acc Chem Res. 2021 Jan 5;54(1):232-241. doi: 10.1021/acs.accounts.0c00651. Epub 2020 Dec 16. Acc Chem Res. 2021. PMID: 33326230
-
Unification of [FeFe]-hydrogenases into three structural and functional groups.Biochim Biophys Acta. 2016 Sep;1860(9):1910-21. doi: 10.1016/j.bbagen.2016.05.034. Epub 2016 May 27. Biochim Biophys Acta. 2016. PMID: 27241847
-
Cyanobacterial hydrogenases and hydrogen metabolism revisited: recent progress and future prospects.Int J Mol Sci. 2015 May 8;16(5):10537-61. doi: 10.3390/ijms160510537. Int J Mol Sci. 2015. PMID: 26006225 Free PMC article. Review.
Cited by
-
Heterologous Hydrogenase Overproduction Systems for Biotechnology-An Overview.Int J Mol Sci. 2020 Aug 16;21(16):5890. doi: 10.3390/ijms21165890. Int J Mol Sci. 2020. PMID: 32824336 Free PMC article. Review.
-
State-of-the-Art Genetic Modalities to Engineer Cyanobacteria for Sustainable Biosynthesis of Biofuel and Fine-Chemicals to Meet Bio-Economy Challenges.Life (Basel). 2019 Jun 27;9(3):54. doi: 10.3390/life9030054. Life (Basel). 2019. PMID: 31252652 Free PMC article. Review.
-
Magnetic Fields as Inducers of Phycobiliprotein Production by Synechococcus elongatus PCC 7942.Curr Microbiol. 2023 Jun 10;80(8):242. doi: 10.1007/s00284-023-03348-3. Curr Microbiol. 2023. PMID: 37300570
-
Co-expression of auxiliary genes enhances the activity of a heterologous O2-tolerant hydrogenase in the cyanobacterium Synechocystis sp. PCC 6803.Biotechnol Biofuels Bioprod. 2025 Mar 28;18(1):41. doi: 10.1186/s13068-025-02634-5. Biotechnol Biofuels Bioprod. 2025. PMID: 40156067 Free PMC article.
-
Cyanobacteria: Photoautotrophic Microbial Factories for the Sustainable Synthesis of Industrial Products.Biomed Res Int. 2015;2015:754934. doi: 10.1155/2015/754934. Epub 2015 Jun 25. Biomed Res Int. 2015. PMID: 26199945 Free PMC article. Review.
References
-
- Ghirardi ML, et al. Hydrogenases and hydrogen photoproduction in oxygenic photosynthetic organisms. Annu Rev Plant Biol. 2007;58:71–91. - PubMed
-
- Vincent KA, Parkin A, Armstrong FA. Investigating and exploiting the electrocatalytic properties of hydrogenases. Chem Rev. 2007;107:4366–4413. - PubMed
-
- Appel J, Phunpruch S, Steinmuller K, Schulz R. The bidirectional hydrogenase of Synechocystis sp. PCC 6803 works as an electron valve during photosynthesis. Arch Microbiol. 2000;173:333–338. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

