Butyrophilin-like 1 encodes an enterocyte protein that selectively regulates functional interactions with T lymphocytes
- PMID: 21368163
- PMCID: PMC3060244
- DOI: 10.1073/pnas.1010647108
Butyrophilin-like 1 encodes an enterocyte protein that selectively regulates functional interactions with T lymphocytes
Abstract
Although local regulation of T-cell responses by epithelial cells is increasingly viewed as important, few molecules mediating such regulation have been identified. Skint1, a recently identified member of the Ig-supergene family expressed by thymic epithelial cells and keratinocytes, specifies the murine epidermal intraepithelial lymphocyte (IEL) repertoire. Investigating whether Skint1-related molecules might regulate IEL in other compartments, this study focuses on buytrophilin-like 1 (Btnl1), which is conspicuously similar to Skint1 and primarily restricted to small intestinal epithelium. Btnl1 protein is mostly cytoplasmic, but surface expression can be induced, and in vivo Btnl1 can be detected adjacent to the IEL. In a newly developed culture system, enforced epithelial cell expression of Btnl1 attenuated the cells' response to activated IEL, as evidenced by suppression of IL-6 and other inflammatory mediators. These findings offer a unique perspective on emerging genetic data that Btnl genes may comprise novel and important local regulators of gut inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
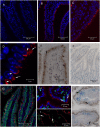
Figures




References
-
- Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–993. - PubMed
-
- Zaph C, et al. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446:552–556. - PubMed
-
- Hayday A, Theodoridis E, Ramsburg E, Shires J. Intraepithelial lymphocytes: Exploring the Third Way in immunology. Nat Immunol. 2001;2:997–1003. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

