Feedback upregulation of HER3 (ErbB3) expression and activity attenuates antitumor effect of PI3K inhibitors
- PMID: 21368164
- PMCID: PMC3286932
- DOI: 10.1073/pnas.1018001108
Feedback upregulation of HER3 (ErbB3) expression and activity attenuates antitumor effect of PI3K inhibitors
Abstract
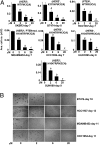
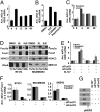
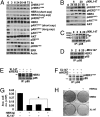
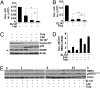
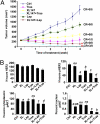
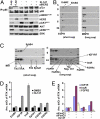
We examined the effects of an inhibitor of PI3K, XL147, against human breast cancer cell lines with constitutive PI3K activation. Treatment with XL147 resulted in dose-dependent inhibition of cell growth and levels of pAKT and pS6, signal transducers in the PI3K/AKT/TOR pathway. In HER2-overexpressing cells, inhibition of PI3K was followed by up-regulation of expression and phosphorylation of multiple receptor tyrosine kinases, including HER3. Knockdown of FoxO1 and FoxO3a transcription factors suppressed the induction of HER3, InsR, IGF1R, and FGFR2 mRNAs upon inhibition of PI3K. In HER2(+) cells, knockdown of HER3 with siRNA or cotreatment with the HER2 inhibitors trastuzumab or lapatinib enhanced XL147-induced cell death and inhibition of pAKT and pS6. Trastuzumab and lapatinib each synergized with XL147 for inhibition of pAKT and growth of established BT474 xenografts. These data suggest that PI3K antagonists will inhibit AKT and relieve suppression of receptor tyrosine kinase expression and their activity. Relief of this feedback limits the sustained inhibition of the PI3K/AKT pathway and attenuates the response to these agents. As a result, PI3K pathway inhibitors may have limited clinical activity overall if used as single agents. In patients with HER2-overexpressing breast cancer, PI3K inhibitors should be used in combination with HER2/HER3 antagonists.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
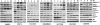
References
-
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. - PubMed
-
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. - PubMed
-
- Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J. Rheb binds and regulates the mTOR kinase. Curr Biol. 2005;15:702–713. - PubMed
-
- Shor B, Gibbons JJ, Abraham RT, Yu K. Targeting mTOR globally in cancer: Thinking beyond rapamycin. Cell Cycle. 2009;8:3831–3837. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

