Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells
- PMID: 21368175
- PMCID: PMC3064359
- DOI: 10.1073/pnas.1017667108
Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells
Erratum in
- Proc Natl Acad Sci U S A. 2011 Oct 18;108(42):17569
Abstract
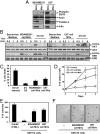
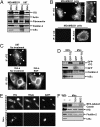
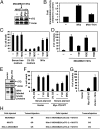
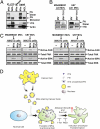
Tumor progression involves the ability of cancer cells to communicate with each other and with neighboring normal cells in their microenvironment. Microvesicles (MV) derived from human cancer cells have received a good deal of attention because of their ability to participate in the horizontal transfer of signaling proteins between cancer cells and to contribute to their invasive activity. Here we show that MV may play another important role in oncogenesis. In particular, we demonstrate that MV shed by two different human cancer cells, MDAMB231 breast carcinoma cells and U87 glioma cells, are capable of conferring onto normal fibroblasts and epithelial cells the transformed characteristics of cancer cells (e.g., anchorage-independent growth and enhanced survival capability) and that this effect requires the transfer of the protein cross-linking enzyme tissue transglutaminase (tTG). We further demonstrate that tTG is not sufficient to transform fibroblasts but rather that it must collaborate with another protein to mediate the transforming actions of the cancer cell-derived MV. Proteomic analyses of the MV derived from MDAMB231 and U87 cells indicated that both these vesicle preparations contained the tTG-binding partner and cross-inking substrate fibronectin (FN). Moreover, we found that tTG cross-links FN in MV from cancer cells and that the ensuing MV-mediated transfers of cross-linked FN and tTG to recipient fibroblasts function cooperatively to activate mitogenic signaling activities and to induce their transformation. These findings highlight a role for MV in the induction of cellular transformation and identify tTG and FN as essential participants in this process.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Al-Nedawi K, Meehan B, Rak J. Microvesicles: Messengers and mediators of tumor progression. Cell Cycle. 2009;8:2014–2018. - PubMed
-
- Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: Artefacts no more. Trends Cell Biol. 2009;19:43–51. - PubMed
-
- Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: Important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20:1487–1495. - PubMed
-
- Al-Nedawi K, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–624. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

