Design and control of acetylcholine receptor conformational change
- PMID: 21368211
- PMCID: PMC3060238
- DOI: 10.1073/pnas.1016617108
Design and control of acetylcholine receptor conformational change
Abstract
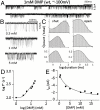
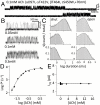
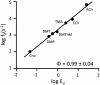
Allosteric proteins use energy derived from ligand binding to promote a global change in conformation. The "gating" equilibrium constant of acetylcholine receptor-channels (AChRs) is influenced by ligands, mutations, and membrane voltage. We engineered AChRs to have specific values of this constant by combining these perturbations, and then calculated the corresponding values for a reference condition. AChRs were designed to have specific rate and equilibrium constants simply by adding multiple, energetically independent mutations with known effects on gating. Mutations and depolarization (to remove channel block) changed the diliganded gating equilibrium constant only by changing the unliganded gating equilibrium constant (E(0)) and did not alter the energy from ligand binding. All of the tested perturbations were approximately energetically independent. We conclude that naturally occurring mutations mainly adjust E(0) and cause human disease because they generate AChRs that have physiologically inappropriate values of this constant. The results suggest that the energy associated with a structural change of a side chain in the gating isomerization is dissipated locally and is mainly independent of rigid body or normal mode motions of the protein. Gating rate and equilibrium constants are estimated for seven different AChR agonists using a stepwise engineering approach.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Edelstein SJ, Changeux JP. Allosteric transitions of the acetylcholine receptor. Adv Protein Chem. 1998;51:121–184. - PubMed
-
- Sine SM, Engel AG. Recent advances in Cys-loop receptor structure and function. Nature. 2006;440:448–455. - PubMed
-
- Dougherty DA. Cys-loop neuroreceptors: Structure to the rescue? Chem Rev. 2008;108:1642–1653. - PubMed
-
- Monod J, Wyman J, Changeux JP. On the nature of allosteric transitions: A plausible model. J Mol Biol. 1965;12:88–118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

