Hedgehog-responsive candidate cell of origin for diffuse intrinsic pontine glioma
- PMID: 21368213
- PMCID: PMC3060250
- DOI: 10.1073/pnas.1101657108
Hedgehog-responsive candidate cell of origin for diffuse intrinsic pontine glioma
Abstract
Diffuse intrinsic pontine gliomas (DIPGs) are highly aggressive tumors of childhood that are almost universally fatal. Our understanding of this devastating cancer is limited by a dearth of available tissue for study and by the lack of a faithful animal model. Intriguingly, DIPGs are restricted to the ventral pons and occur during a narrow window of middle childhood, suggesting dysregulation of a postnatal neurodevelopmental process. Here, we report the identification of a previously undescribed population of immunophenotypic neural precursor cells in the human and murine brainstem whose temporal and spatial distributions correlate closely with the incidence of DIPG and highlight a candidate cell of origin. Using early postmortem DIPG tumor tissue, we have established in vitro and xenograft models and find that the Hedgehog (Hh) signaling pathway implicated in many developmental and oncogenic processes is active in DIPG tumor cells. Modulation of Hh pathway activity has functional consequences for DIPG self-renewal capacity in neurosphere culture. The Hh pathway also appears to be active in normal ventral pontine precursor-like cells of the mouse, and unregulated pathway activity results in hypertrophy of the ventral pons. Together, these findings provide a foundation for understanding the cellular and molecular origins of DIPG, and suggest that the Hh pathway represents a potential therapeutic target in this devastating pediatric tumor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

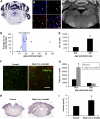


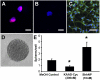
References
-
- Central Brain Tumor Registry of the United States . CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004-2006. Hinsdale, IL: Central Brain Tumor Registry of the United States; 2010.
-
- Barkovich AJ, et al. Brain stem gliomas: A classification system based on magnetic resonance imaging. Pediatr Neurosurg. 1990-1991;16:73–83. - PubMed
-
- Fisher PG, et al. A clinicopathologic reappraisal of brain stem tumor classification. Identification of pilocystic astrocytoma and fibrillary astrocytoma as distinct entities. Cancer. 2000;89:1569–1576. - PubMed
-
- Donaldson SS, Laningham F, Fisher PG. Advances toward an understanding of brainstem gliomas. J Clin Oncol. 2006;24:1266–1272. - PubMed
-
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

