Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes
- PMID: 21368230
- PMCID: PMC3091943
- DOI: 10.4049/jimmunol.1003099
Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes
Abstract
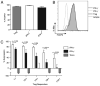
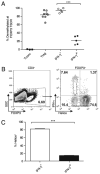
Regulatory T cells (Tregs) constitute an attractive therapeutic target given their essential role in controlling autoimmunity. However, recent animal studies provide evidence for functional heterogeneity and lineage plasticity within the Treg compartment. To understand better the plasticity of human Tregs in the context of type 1 diabetes, we characterized an IFN-γ-competent subset of human CD4(+)CD127(lo/-)CD25(+) Tregs. We measured the frequency of Tregs in the peripheral blood of patients with type 1 diabetes by epigenetic analysis of the Treg-specific demethylated region (TSDR) and the frequency of the IFN-γ(+) subset by flow cytometry. Purified IFN-γ(+) Tregs were assessed for suppressive function, degree of TSDR demethylation, and expression of Treg lineage markers FOXP3 and Helios. The frequency of Tregs in peripheral blood was comparable but the FOXP3(+)IFN-γ(+) fraction was significantly increased in patients with type 1 diabetes compared to healthy controls. Purified IFN-γ(+) Tregs expressed FOXP3 and possessed suppressive activity but lacked Helios expression and were predominately methylated at the TSDR, characteristics of an adaptive Treg. Naive Tregs were capable of upregulating expression of Th1-associated T-bet, CXCR3, and IFN-γ in response to IL-12. Notably, naive, thymic-derived natural Tregs also demonstrated the capacity for Th1 differentiation without concomitant loss of Helios expression or TSDR demethylation.
Figures




References
-
- Bach JF. Autoimmune diseases as the loss of active “self-control”. Ann NY Acad Sci. 2003;998:161–177. - PubMed
-
- Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358:221–229. - PubMed
-
- Brusko TM, Putnam AL, Bluestone JA. Human regulatory T cells: role in autoimmune disease and therapeutic opportunities. Immunol Rev. 2008;223:371–390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

