Simian immunodeficiency virus infection in the brain and lung leads to differential type I IFN signaling during acute infection
- PMID: 21368232
- PMCID: PMC3076806
- DOI: 10.4049/jimmunol.1003757
Simian immunodeficiency virus infection in the brain and lung leads to differential type I IFN signaling during acute infection
Abstract
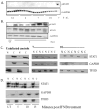
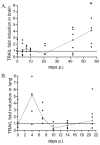
Using an accelerated and consistent SIV pigtailed macaque model of HIV-associated neurologic disorders, we have demonstrated that virus enters the brain during acute infection. However, neurologic symptoms do not manifest until late stages of infection, suggesting that immunological mechanisms exist within the CNS that control viral replication and associated inflammation. We have shown that IFN-β, a type I IFN central to viral innate immunity, is a major cytokine present in the brain during acute infection and is responsible for limiting virus infection and inflammatory cytokine expression. However, the induction and role of IFN-α in the CNS during acute SIV infection has never been examined in this model. In the classical model of IFN signaling, IFN-β signals through the IFN-α/β receptor, leading to expression of IFN-α. Surprisingly, although IFN-β is upregulated during acute SIV infection, we found that IFN-α is downregulated. We demonstrate that this downregulation is coupled with a suppression of signaling molecules downstream of the IFN receptor, namely tyrosine kinase 2, STAT1, and IFN regulatory factor 7, as indicated by either lack of protein phosphorylation, lack of nuclear accumulation, or transcriptional and/or translational repression. In contrast to brain, IFN-α is upregulated in lung and accompanied by activation of tyrosine kinase 2 and STAT1. These data provide a novel observation that during acute SIV infection in the brain, there is differential signaling through the IFN-α/β receptor that fails to activate expression of IFN-α in the brain.
Figures




References
-
- McArthur JC, Haughey N, Gartner S, Conant K, Pardo C, Nath A, Sacktor N. Human immunodeficiency virus-associated dementia: an evolving disease. J Neurovirol. 2003;9:205–221. - PubMed
-
- Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, Stern Y, Albert S, Palumbo D, Kieburtz K, De Marcaida JA, Cohen B, Epstein L. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8:136–142. - PubMed
-
- Fischer-Smith T, Rappaport J. Evolving paradigms in the pathogenesis of HIV-1-associated dementia. Expert Rev Mol Med. 2005;7:1–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

