Neuromyelitis optica unique area postrema lesions: nausea, vomiting, and pathogenic implications
- PMID: 21368286
- PMCID: PMC3068006
- DOI: 10.1212/WNL.0b013e318214332c
Neuromyelitis optica unique area postrema lesions: nausea, vomiting, and pathogenic implications
Abstract
Objective: To characterize the neuropathologic features of neuromyelitis optica (NMO) at the medullary floor of the fourth ventricle and area postrema. Aquaporin-4 (AQP4) autoimmunity targets this region, resulting in intractable nausea associated with vomiting or hiccups in NMO.
Methods: This neuropathologic study was performed on archival brainstem tissue from 15 patients with NMO, 5 patients with multiple sclerosis (MS), and 8 neurologically normal subjects. Logistic regression was used to evaluate whether the presence of lesions at this level increased the odds of a patient with NMO having an episode of nausea/vomiting.
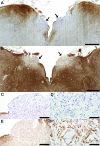
Results: Six patients with NMO (40%), but no patients with MS or normal controls, exhibited unilateral or bilateral lesions involving the area postrema and the medullary floor of the fourth ventricle. These lesions were characterized by tissue rarefaction, blood vessel thickening, no obvious neuronal or axonal pathology, and preservation of myelin in the subependymal medullary tegmentum. AQP4 immunoreactivity was lost or markedly reduced in all 6 cases, with moderate to marked perivascular and parenchymal lymphocytic inflammatory infiltrates, prominent microglial activation, and in 3 cases, eosinophils. Complement deposition in astrocytes, macrophages, and/or perivascularly, and a prominent astroglial reaction were also present. The odds of nausea/vomiting being documented clinically was 16-fold greater in NMO cases with area postrema lesions (95% confidence interval 1.43-437, p = 0.02).
Conclusions: These neuropathologic findings suggest the area postrema may be a selective target of the disease process in NMO, and are compatible with clinical reports of nausea and vomiting preceding episodes of optic neuritis and transverse myelitis or being the heralding symptom of NMO.
Figures



Comment in
-
Neuromyelitis optica: circulating autoantibodies provoke astrocyte-mediated neural dysfunction.Neurology. 2011 Apr 5;76(14):1202-3. doi: 10.1212/WNL.0b013e3182143355. Epub 2011 Mar 2. Neurology. 2011. PMID: 21368284 No abstract available.
References
-
- Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology 2006;66:1485–1489 - PubMed
-
- Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol 2007;6:805–815 - PubMed
-
- Pittock SJ, Lennon VA, Krecke K, Wingerchuk DM, Lucchinetti CF, Weinshenker BG. Brain abnormalities in neuromyelitis optica. Arch Neurol 2006;63:390–396 - PubMed
-
- Pittock SJ, Weinshenker BG, Lucchinetti CF, Wingerchuk DM, Corboy JR, Lennon VA. Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch Neurol 2006;63:964–968 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
