Dysregulation of Ca2+ signaling in astrocytes from mice lacking amyloid precursor protein
- PMID: 21368296
- PMCID: PMC3118622
- DOI: 10.1152/ajpcell.00379.2010
Dysregulation of Ca2+ signaling in astrocytes from mice lacking amyloid precursor protein
Abstract
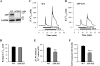
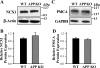
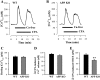
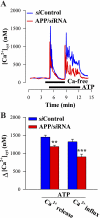
The relationship between altered metabolism of the amyloid-β precursor protein (APP) and Alzheimer's disease is well established but the physiological roles of APP still remain unclear. Here, we studied Ca(2+) signaling in primary cultured and freshly dissociated cortical astrocytes from APP knockout (KO) mice and from Tg5469 mice overproducing by five- to sixfold wild-type APP. Resting cytosolic Ca(2+) (measured with fura-2) was not altered in cultured astrocytes from APP KO mice. The stored Ca(2+) evaluated by measuring peak amplitude of cyclopiazonic acid [CPA, endoplasmic reticulum (ER) Ca(2+) ATPase inhibitor]-induced Ca(2+) transients in Ca(2+)-free medium was significantly smaller in APP KO astrocytes than in wild-type cells. Store-operated Ca(2+) entry (SOCE) activated by ER Ca(2+) store depletion with CPA was also greatly reduced in APP KO astrocytes. This reflected a downregulated expression in APP KO astrocytes of TRPC1 (C-type transient receptor potential) and Orai1 proteins, essential components of store-operated channels (SOCs). Indeed, silencer RNA (siRNA) knockdown of Orai1 protein expression in wild-type astrocytes significantly attenuated SOCE. SOCE was also essentially reduced in freshly dissociated APP KO astrocytes. Importantly, knockdown of APP with siRNA in cultured wild-type astrocytes markedly attenuated ATP- and CPA-induced ER Ca(2+) release and extracellular Ca(2+) influx. The latter correlated with downregulation of TRPC1. Overproduction of APP in Tg5469 mice did not alter, however, the stored Ca(2+) level, SOCE, and expression of TRPC1/4/5 in cultured astrocytes from these mice. The data demonstrate that the functional role of APP in astrocytes involves the regulation of TRPC1/Orai1-encoded SOCs critical for Ca(2+) signaling.
Figures



Similar articles
-
Histidine triad nucleotide-binding protein 1 (HINT1) regulates Ca(2+) signaling in mouse fibroblasts and neuronal cells via store-operated Ca(2+) entry pathway.Am J Physiol Cell Physiol. 2013 Jun 1;304(11):C1098-104. doi: 10.1152/ajpcell.00073.2013. Epub 2013 Apr 10. Am J Physiol Cell Physiol. 2013. PMID: 23576580 Free PMC article.
-
Local Ca²+ entry via Orai1 regulates plasma membrane recruitment of TRPC1 and controls cytosolic Ca²+ signals required for specific cell functions.PLoS Biol. 2011 Mar;9(3):e1001025. doi: 10.1371/journal.pbio.1001025. Epub 2011 Mar 8. PLoS Biol. 2011. PMID: 21408196 Free PMC article.
-
Mechanisms of interleukin-1beta-induced Ca2+ signals in mouse cortical astrocytes: roles of store- and receptor-operated Ca2+ entry.Am J Physiol Cell Physiol. 2007 Sep;293(3):C1103-11. doi: 10.1152/ajpcell.00249.2007. Epub 2007 Aug 1. Am J Physiol Cell Physiol. 2007. PMID: 17670890
-
STIM-TRP Pathways and Microdomain Organization: Contribution of TRPC1 in Store-Operated Ca2+ Entry: Impact on Ca2+ Signaling and Cell Function.Adv Exp Med Biol. 2017;993:159-188. doi: 10.1007/978-3-319-57732-6_9. Adv Exp Med Biol. 2017. PMID: 28900914 Review.
-
Fine-tuning of store-operated calcium entry by fast and slow Ca2+-dependent inactivation: Involvement of SARAF.Biochim Biophys Acta Mol Cell Res. 2018 Mar;1865(3):463-469. doi: 10.1016/j.bbamcr.2017.12.001. Epub 2017 Dec 6. Biochim Biophys Acta Mol Cell Res. 2018. PMID: 29223474 Review.
Cited by
-
TRP channels coordinate ion signalling in astroglia.Rev Physiol Biochem Pharmacol. 2014;166:1-22. doi: 10.1007/112_2013_15. Rev Physiol Biochem Pharmacol. 2014. PMID: 23784619 Free PMC article. Review.
-
"TRP inflammation" relationship in cardiovascular system.Semin Immunopathol. 2016 May;38(3):339-56. doi: 10.1007/s00281-015-0536-y. Epub 2015 Oct 19. Semin Immunopathol. 2016. PMID: 26482920 Free PMC article. Review.
-
The Role of APP in Structural Spine Plasticity.Front Mol Neurosci. 2017 May 10;10:136. doi: 10.3389/fnmol.2017.00136. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28539872 Free PMC article. Review.
-
STIM and ORAI proteins in the nervous system.Channels (Austin). 2015;9(5):245-52. doi: 10.1080/19336950.2015.1071747. Epub 2015 Jul 28. Channels (Austin). 2015. PMID: 26218135 Free PMC article. Review.
-
Functional Importance of Transient Receptor Potential (TRP) Channels in Neurological Disorders.Front Cell Dev Biol. 2021 Mar 4;9:611773. doi: 10.3389/fcell.2021.611773. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33748103 Free PMC article. Review.
References
-
- Araque A, Sanzgiri RP, Parpura V, Haydon PG. Astrocyte-induced modulation of synaptic transmission. Can J Physiol Pharmacol 77: 699–706, 1999 - PubMed
-
- Bambrick LL, de Grip A, Seenivasan V, Krueger BK, Yarowsky PJ. Expression of glial antigens in mouse astrocytes: species differences and regulation in vitro. J Neurosci Res 46: 305–315, 1996 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

