Enhancer of Zeste homolog 2 (EZH2) is overexpressed in recurrent nasopharyngeal carcinoma and is regulated by miR-26a, miR-101, and miR-98
- PMID: 21368858
- PMCID: PMC3035896
- DOI: 10.1038/cddis.2010.64
Enhancer of Zeste homolog 2 (EZH2) is overexpressed in recurrent nasopharyngeal carcinoma and is regulated by miR-26a, miR-101, and miR-98
Abstract
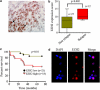
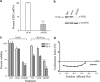
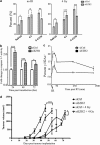
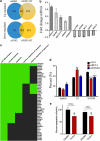
There is increasing evidence supporting the role of members of the polycomb group (PcG) gene family in tumor development and progression. However, their precise role in tumorigenesis and mechanisms of their regulation remain to be elucidated. Using nasopharyngeal carcinoma (NPC) as a disease model, a comprehensive analysis was undertaken on the clinical significance of EZH2 expression, identification of the cellular processes regulated by EZH2, and the mechanisms of its deregulated expression. Herein, we report EZH2 as being associated with a higher risk of relapse in NPC patients (P = 0.002). Genome-wide microarray and bioinformatics identified several vital cellular processes (such as differentiation, development, and apoptosis) to be regulated by EZH2, corroborated by in vitro lethality, and delayed tumor formation in vivo upon EZH2 depletion. The combination of global microRNA (miR) profiling in primary NPC specimens, and in silico analyses provided several candidate miRs that could regulate EZH2. Using a luciferase-based assay, miR-26a, miR-101, and miR-98 were validated as bona fide regulators of EZH2 expression. In particular, miR-98 was underexpressed in relapsed patient samples, strongly suggesting an important role for the miR-98 and EZH2 axis in NPC biology.
Figures

References
-
- Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419:624–629. - PubMed
-
- Villa R, Pasini D, Gutierrez A, Morey L, Occhionorelli M, Vire E, et al. Role of the polycomb repressive complex 2 in acute promyelocytic leukemia. Cancer Cell. 2007;11:513–525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

