Palmitoylation of human FasL modulates its cell death-inducing function
- PMID: 21368861
- PMCID: PMC3035908
- DOI: 10.1038/cddis.2010.62
Palmitoylation of human FasL modulates its cell death-inducing function
Abstract
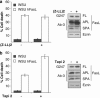
Fas ligand (FasL) is a transmembrane protein that regulates cell death in Fas-bearing cells. FasL-mediated cell death is essential for immune system homeostasis and the elimination of viral or transformed cells. Because of its potent cytotoxic activity, FasL expression at the cell surface is tightly regulated, for example, via processing by ADAM10 and SPPL2a generating soluble FasL and the intracellular fragments APL (ADAM10-processed FasL form) and SPA (SPPL2a-processed APL). In this study, we report that FasL processing by ADAM10 counteracts Fas-mediated cell death and is strictly regulated by membrane localization, interactions and modifications of FasL. According to our observations, FasL processing occurs preferentially within cholesterol and sphingolipid-rich nanodomains (rafts) where efficient Fas-FasL contact occurs, Fas receptor and FasL interaction is also required for efficient FasL processing, and FasL palmitoylation, which occurs within its transmembrane domain, is critical for efficient FasL-mediated killing and FasL processing.
Figures


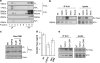

Similar articles
-
The Fas ligand intracellular domain is released by ADAM10 and SPPL2a cleavage in T-cells.Cell Death Differ. 2007 Sep;14(9):1678-87. doi: 10.1038/sj.cdd.4402175. Epub 2007 Jun 8. Cell Death Differ. 2007. PMID: 17557115
-
ADAM10 regulates FasL cell surface expression and modulates FasL-induced cytotoxicity and activation-induced cell death.Cell Death Differ. 2007 May;14(5):1040-9. doi: 10.1038/sj.cdd.4402101. Epub 2007 Feb 9. Cell Death Differ. 2007. PMID: 17290285
-
Fas/FasL-dependent and -independent activation of caspase-8 in doxorubicin-treated human breast cancer MCF-7 cells: ADAM10 down-regulation activates Fas/FasL signaling pathway.Int J Biochem Cell Biol. 2011 Dec;43(12):1708-19. doi: 10.1016/j.biocel.2011.08.004. Epub 2011 Aug 12. Int J Biochem Cell Biol. 2011. PMID: 21854868
-
Storage, expression and function of Fas ligand, the key death factor of immune cells.Curr Med Chem. 2008;15(17):1684-96. doi: 10.2174/092986708784872384. Curr Med Chem. 2008. PMID: 18673218 Review.
-
The FasLane to ocular pathology-metalloproteinase cleavage of membrane-bound FasL determines FasL function.J Leukoc Biol. 2021 Nov;110(5):965-977. doi: 10.1002/JLB.3RI1220-834R. Epub 2021 Feb 10. J Leukoc Biol. 2021. PMID: 33565149 Review.
Cited by
-
Protein S-palmitoylation in immunity.Open Biol. 2021 Mar;11(3):200411. doi: 10.1098/rsob.200411. Epub 2021 Mar 3. Open Biol. 2021. PMID: 33653086 Free PMC article. Review.
-
Palmitoylation is required for TNF-R1 signaling.Cell Commun Signal. 2019 Aug 5;17(1):90. doi: 10.1186/s12964-019-0405-8. Cell Commun Signal. 2019. PMID: 31382980 Free PMC article.
-
ADAM10-a "multitasker" in sepsis: focus on its posttranslational target.Inflamm Res. 2023 Mar;72(3):395-423. doi: 10.1007/s00011-022-01673-0. Epub 2022 Dec 24. Inflamm Res. 2023. PMID: 36565333 Free PMC article. Review.
-
Lipid rafts as signaling hubs in cancer cell survival/death and invasion: implications in tumor progression and therapy: Thematic Review Series: Biology of Lipid Rafts.J Lipid Res. 2020 May;61(5):611-635. doi: 10.1194/jlr.TR119000439. Epub 2020 Nov 7. J Lipid Res. 2020. PMID: 33715811 Free PMC article. Review.
-
CD95L and Anti-Tumor Immune Response: Current Understanding and New Evidence.Cancer Manag Res. 2021 Mar 16;13:2477-2482. doi: 10.2147/CMAR.S297499. eCollection 2021. Cancer Manag Res. 2021. PMID: 33758545 Free PMC article.
References
-
- Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–1192. - PubMed
-
- Ferguson TA, Green DR, Griffith TS. Cell death and immune privilege. Int Rev Immunol. 2002;21:153–172. - PubMed
-
- Krammer PH. The tumor strikes back: new data on expression of the CD95(APO-1/Fas) receptor/ligand system may cause paradigm changes in our view on drug treatment and tumor immunology. Cell Death Differ. 1997;4:362–364. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

