Cytotoxicity of amyloidogenic immunoglobulin light chains in cell culture
- PMID: 21368874
- PMCID: PMC3032327
- DOI: 10.1038/cddis.2010.75
Cytotoxicity of amyloidogenic immunoglobulin light chains in cell culture
Abstract
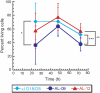
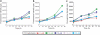
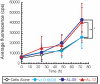
Light-chain amyloidosis (AL) is a devastating protein-misfolding disease characterized by abnormal proliferation of plasma cells in the bone marrow that secrete monoclonal immunoglobulin light chains that misfold and form amyloid fibrils, thus causing organ failure and death. Numerous reports on different protein-misfolding diseases show that soluble oligomeric species populated by amyloidogenic proteins can be quite toxic to cells. However, it is not well established whether the soluble immunoglobulin light-chain species found in circulation in patients with AL are toxic to cells in target organs. We determined the cellular toxicity of two well-characterized light-chain variable domain proteins from cardiac AL patients and their corresponding germline protein, devoid of somatic mutations. Our results show that the soluble form of the AL proteins we characterized are toxic to cardiomyocytes, and that the species found in cell culture correspond, for the most part, to the species present in circulation in these patients.
Figures


References
-
- Harper JD, Wong SS, Lieber CM, Lansbury PT. Observation of metastable Abeta amyloid protofibrils by atomic force microscopy. Chem Biol. 1997;4:119–125. - PubMed
-
- Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, et al. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. - PubMed
-
- Pokrzywa M, Dacklin I, Hultmark D, Lundgren E. Misfolded transthyretin causes behavioral changes in a Drosophila model for transthyretin-associated amyloidosis. Eur J Neurosci. 2007;26:913–924. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

