Common structural traits for cystine knot domain of the TGFβ superfamily of proteins and three-fingered ectodomain of their cellular receptors
- PMID: 21369710
- PMCID: PMC11114550
- DOI: 10.1007/s00018-011-0643-4
Common structural traits for cystine knot domain of the TGFβ superfamily of proteins and three-fingered ectodomain of their cellular receptors
Abstract
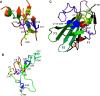
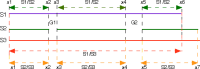
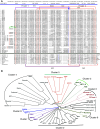
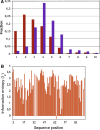
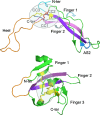
The transforming growth factor-β (TGFβ) superfamily of proteins and their receptors are crucial developmental factors for all metazoan organisms. Cystine-knot (CK) motif is a spatial feature of the TGFβ superfamily of proteins whereas the extra-cellular domains (ectodomains) of their respective receptors form three-fingered protein domain (TFPD), both stabilized by tight cystine networks. Analyses of multiple sequence alignments of these two domains encoded in various genomes revealed that the cystines forming the CK and TFPD folds are conserved, whereas the remaining polypeptide patches are diversified. Orthologues of the human TGFβs and their respective receptors expressed in diverse vertebrates retain high sequence conservation. Examination of 3D structures of various TGFβ factors bound to their receptors have revealed that the CK and TFPD domains display several similar spatial traits suggesting that these two different protein folds might have been acquired from a common ancestor.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

