One-day versus 3-day suprapubic catheterization after vaginal prolapse surgery: a prospective randomized trial
- PMID: 21369817
- PMCID: PMC3072491
- DOI: 10.1007/s00192-011-1358-7
One-day versus 3-day suprapubic catheterization after vaginal prolapse surgery: a prospective randomized trial
Abstract
Introduction and hypothesis: For prolonged catheterization after vaginal prolapse surgery with anterior colporrhaphy, the optimal duration to prevent overdistention of the bladder remains unknown. We designed this study to determine the optimal length of catheterization.
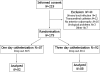
Methods: We conducted a prospective randomized trial in which 179 women were allocated to 1-day or 3-day suprapubic catheterization. The primary outcome was the duration of catheterization.
Results: Mean duration of catheterization and hospital stay was significantly shorter in the 1-day catheterization group. The number of successful voiding trials was higher in the 3-day catheterization group (90.9% versus 79.3%), but this did not reach statistical significance. The percentage of urinary tract infection did not differ significantly between the groups (4.5% versus 2.4%).
Conclusion: Starting a voiding trial 1 day after vaginal prolapse surgery leads to shorter duration of catheterization and hospital stay.
Figures
References
-
- Glavind K, Mørup L, Madsen H, Glavind J. A prospective, randomised, controlled trial comparing 3 hour and 24 hour postoperative removal of bladder catheter and vaginal pack following vaginal prolapse surgery. Acta Obstet Gynecol Scand. 2007;86:1122–1125. doi: 10.1080/00016340701505317. - DOI - PubMed
-
- Niël-Weise BS, van den Broek PJ. Urinary catheter policies for short-term bladder drainage in adults. Cochrane Database Syst Rev. 2005 - PubMed
-
- Hakvoort RA, Elberink R, Vollebregt A, Van der Ploeg T, Emanuel MH. How long should bladder catheterisation be continued after vaginal prolapse surgery? a randomised controlled trial comparing short term versus long term catheterisation after vaginal prolapse surgery. BJOG. 2004;111:828–830. doi: 10.1111/j.1471-0528.2004.00181.x. - DOI - PubMed