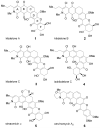
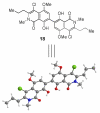
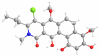
Synthesis and biological evaluation of ABCD ring fragments of the kibdelones
- PMID: 21370327
- PMCID: PMC3376643
- DOI: 10.1002/anie.201007613
Synthesis and biological evaluation of ABCD ring fragments of the kibdelones
Figures
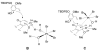
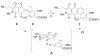



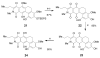
References
-
- Lee TM, Carter GT, Borders DB. J. Chem. Soc. Chem. Commun. 1989;22:1771–1772.
- Carter GT, Goodman JJ, Torrey MJ, Borders DB, Gould SJ. J. Org. Chem. 1989;54:4321–4323.
- Maiese WM, Korshalla J, Goodman J, Torrey MJ, Kantor S, Labeda DP, Greenstein M. J. Antibiotics. 1990;43:1059–1063. - PubMed
- Arai M, Sato H, Kobayashi H, Suganuma M, Kawabe T, Tomoda H, Omura S. Biochem. Biophys. Res. Comm. 2004;317:817–822. - PubMed
-
-
cervinomycin A2: Omura S, Nakagawa A, Kushida K, Shimizu H, Lukacs GJ. Antibiot. 1987;40:301–308. Tanka H, Kawakita K, Suzuki H, Nakagawa PS, Omura SJ. J. Antibiot. 1989;42:431–439. Mehta G, Venkateswarlu J. Chem Soc., Chem. Commun. 1988:1200–1202. Kelly TR, Jagoe CT, Li Q. J. Am. Chem. Soc. 1989;111:4522–4524. Rao AV, Yadav JS, Reddy K, Upender V. Tetrahedron Lett. 1991;32:5199–5202. Yadav JS. Pure Appl. Chem. 1993;65:1349–1356. Mehta G, Shah SR, Venkateswarlu Y. Tetrahedron. 1994;50:11729–11742. lysolipin. Duthaler RO, Wegmann UH. Helv. Chim. Acta. 1984;67:1755–11766. Walker ER, Leung SY, Barrett AGM. Tetrahedron Lett. 2005;46:6537–6540. Sch 56036.
-
-
- Manning WB, Kelly TP, Muschik GM. J. Org. Chem. 1980;45:2535.
- Muschik GM, Kelly TP, Manning WB. J. Org. Chem. 1982;47:4709.
- Tanga MJ, Reist EJ. J. Heterocycl. Chem. 1991:28–29.
- Willmore ND, Hoic DA, Katz TJ. J. Org. Chem. 1994;59:1889.
- Carreño MC, Mahugo J, Urbano A. Tetrahedron Lett. 1997;38:3047.
- Katz TJ, Liu L, Willmore ND, Fox JM, Rheingold AL, Shi S, Nuckolls C, Rickman BH. J. Am. Chem. Soc. 1997;119:10054.
- Krohn K, Loock U, Paavilainen K, Hausen BM, Schmalle WH, Kiesele H. ARKIVOC. 2001;2:88–130.
-
- Fisher MJ, Hehre WJ, Kahn SD, Overman LE. J. Am. Chem. Soc. 1988;110:4625.
- Tripathy R, Carroll PJ, Thornton ER. J. Am. Chem. Soc. 1990;112:6743.
- Sorgi KL, McNally JJ, Leo GC. J. Org. Chem. 1994;59:5088.
- Atherton JCC, Jones S. Tetrahedron Lett. 2001;42:8239.
- Huang Y, Rawal VH. J. Am. Chem. Soc. 2002;124:9662. - PubMed
- Domingo LR, Aurell MJ, Arno M, Saez JA. J. Org. Chem. 2007;72:4220–4227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

