Chronic treatment with angiotensin-(1-7) improves renal endothelial dysfunction in apolipoproteinE-deficient mice
- PMID: 21371005
- PMCID: PMC3130944
- DOI: 10.1111/j.1476-5381.2011.01295.x
Chronic treatment with angiotensin-(1-7) improves renal endothelial dysfunction in apolipoproteinE-deficient mice
Abstract
Background and purpose: ApolipoproteinE-deficient [apoE (-/-)] mice, a model of human atherosclerosis, develop endothelial dysfunction caused by decreased levels of nitric oxide (NO). The endogenous peptide, angiotensin-(1-7) [Ang-(1-7)], acting through its specific GPCR, the Mas receptor, has endothelium-dependent vasodilator properties. Here we have investigated if chronic treatment with Ang-(1-7) improved endothelial dysfunction in apoE (-/-) mice.
Experimental approach: ApoE (-/-) mice fed on a lipid-rich Western diet were divided into three groups and treated via osmotic minipumps with either saline, Ang-(1-7) (82 µg·kg(-1) ·h(-1) ) or the same dose of Ang-(1-7) together with D-Ala-Ang-(1-7) (125 µg·kg(-1) ·h(-1) ) for 6 weeks. Renal vascular function was assessed in isolated perfused kidneys.
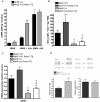
Key results: Ang-(1-7)-treated apoE (-/-) mice showed improved renal endothelium-dependent vasorelaxation induced by carbachol and increased renal basal cGMP production, compared with untreated apoE (-/-) mice. Tempol, a reactive oxygen species (ROS) scavenger, improved endothelium-dependent vasorelaxation in kidneys of saline-treated apoE (-/-) mice whereas no effect was observed in Ang-(1-7)-treated mice. Chronic treatment with D-Ala-Ang-(1-7), a specific Mas receptor antagonist, abolished the beneficial effects of Ang-(1-7) on endothelium-dependent vasorelaxation. Renal endothelium-independent vasorelaxation showed no differences between treated and untreated mice. ROS production and expression levels of the NAD(P)H oxidase subunits gp91phox and p47phox were reduced in isolated preglomerular arterioles of Ang-(1-7)-treated mice, compared with untreated mice, whereas eNOS expression was increased.
Conclusion and implications: Chronic infusion of Ang-(1-7) improved renal endothelial function via Mas receptors, in an experimental model of human cardiovascular disease, by increasing levels of endogenous NO.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures


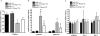
References
-
- Arruda RM, Peotta VA, Meyrelles SS, Vasquez EC. Evaluation of vascular function in apolipoprotein E knockout mice with angiotensin-dependent renovascular hypertension. Hypertension. 2005;46:932–936. - PubMed
-
- Benter IF, Yousif MH, Dhaunsi GS, Kaur J, Chappell MC, Diz DI. Angiotensin-(1-7) prevents activation of NADPH oxidase and renal vascular dysfunction in diabetic hypertensive rats. Am J Nephrol. 2008;28:25–33. - PubMed
-
- Brooker G, Harper JF, Terasaki WL, Moylan RD. Radioimmunoassay of cyclic AMP and cyclic GMP. Adv Cyclic Nucleotide Res. 1979;10:1–33. - PubMed
-
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

