Neisseria gonorrhoeae pilin glycan contributes to CR3 activation during challenge of primary cervical epithelial cells
- PMID: 21371235
- PMCID: PMC3889163
- DOI: 10.1111/j.1462-5822.2011.01586.x
Neisseria gonorrhoeae pilin glycan contributes to CR3 activation during challenge of primary cervical epithelial cells
Abstract
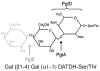
Expression of type IV pili by Neisseria gonorrhoeae plays a critical role in mediating adherence to human epithelial cells. Gonococcal pilin is modified with an O-linked glycan, which may be present as a di- or monosaccharide because of phase variation of select pilin glycosylation genes. It is accepted that bacterial proteins may be glycosylated; less clear is how the protein glycan may mediate virulence. Using primary, human, cervical epithelial (i.e. pex) cells, we now provide evidence to indicate that the pilin glycan mediates productive cervical infection. In this regard, pilin glycan-deficient mutant gonococci exhibited an early hyper-adhesive phenotype but were attenuated in their ability to invade pex cells. Our data further indicate that the pilin glycan was required for gonococci to bind to the I-domain region of complement receptor 3, which is naturally expressed by pex cells. Comparative, quantitative, infection assays revealed that mutant gonococci lacking the pilin glycan did not bind to the I-domain when it is in a closed, low-affinity conformation and cannot induce an active conformation to complement receptor 3 during pex cell challenge. To our knowledge, these are the first data to directly demonstrate how a protein-associated bacterial glycan may contribute to pathogenesis.
© 2011 Blackwell Publishing Ltd.
Figures






References
-
- Aas FE, Egge-Jacobsen W, Winther-Larsen HC, Løvold C, Hitchen PG, Dell A, Koomey M. Neisseria gonorrhoeae type IV pili undergo multisite, hierarchial modifications with phosphoethanolamine and phosphocho-line requiring an enzyme structurally related to lipopolysaccharide phosphoethanolamine transferases. J Biol Chem. 2006;281:27712–27723. - PubMed
-
- Aota SI, Nagai T, Yamada KM. Characterization of regions of fibronectin besides the arginine-glycine-aspartic acid sequence required for adhesive function of the cell-binding domain using site-directed mutagenesis. J Biol Chem. 1991;266:15938–15943. - PubMed
-
- Aota SI, Nomizu M, Yamada KM. The short amino acid sequence pro-his-ser-arg-asn in human fibronectin enhances cell-adhesive function. J Biol Chem. 1994;269:24756–24761. - PubMed
-
- Apicella MA. Antigenically distinct populations of Neisseria gonorrhoeae: isolation and characterization of the responsible determinants. J Infect Dis. 1974;130:619–625. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

