Progesterone inhibition of voltage-gated calcium channels is a potential neuroprotective mechanism against excitotoxicity
- PMID: 21371490
- PMCID: PMC3129396
- DOI: 10.1016/j.steroids.2011.02.013
Progesterone inhibition of voltage-gated calcium channels is a potential neuroprotective mechanism against excitotoxicity
Abstract
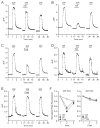
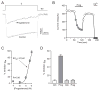
The therapeutic use of progesterone following traumatic brain injury has recently entered phase III clinical trials as a means of neuroprotection. Although it has been hypothesized that progesterone protects against calcium overload following excitotoxic shock, the exact mechanisms underlying the beneficial effects of progesterone have yet to be determined. We found that therapeutic concentrations of progesterone to be neuroprotective against depolarization-induced excitotoxicity in cultured striatal neurons. Through use of calcium imaging, electrophysiology and the measurement of changes in activity-dependent gene expression, progesterone was found to block calcium entry through voltage-gated calcium channels, leading to alterations in the signaling of the activity-dependent transcription factors NFAT and CREB. The effects of progesterone were highly specific to this steroid hormone, although they did not appear to be receptor mediated. In addition, progesterone did not inhibit AMPA or NMDA receptor signaling. This analysis regarding the effect of progesterone on calcium signaling provides both a putative mechanism by which progesterone acts as a neuroprotectant, as well as affords a greater appreciation for its potential far-reaching effects on cellular function.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
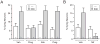
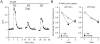
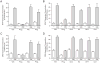
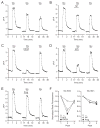
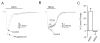
Similar articles
-
Progesterone inhibition of neuronal calcium signaling underlies aspects of progesterone-mediated neuroprotection.J Steroid Biochem Mol Biol. 2012 Aug;131(1-2):30-6. doi: 10.1016/j.jsbmb.2011.11.002. Epub 2011 Nov 12. J Steroid Biochem Mol Biol. 2012. PMID: 22101209 Free PMC article. Review.
-
Excitotoxic death of a subset of embryonic rat motor neurons in vitro.J Neurochem. 1999 Feb;72(2):500-13. doi: 10.1046/j.1471-4159.1999.0720500.x. J Neurochem. 1999. PMID: 9930721
-
α-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid receptor activation protects against phencyclidine-induced caspase-3 activity by activating voltage-gated calcium channels.J Neurosci Res. 2014 Dec;92(12):1785-91. doi: 10.1002/jnr.23446. Epub 2014 Jul 4. J Neurosci Res. 2014. PMID: 24995437
-
Ca(2+) influx through AMPA or kainate receptors alone is sufficient to initiate excitotoxicity in cultured oligodendrocytes.Neurobiol Dis. 2002 Mar;9(2):234-43. doi: 10.1006/nbdi.2001.0457. Neurobiol Dis. 2002. PMID: 11895374
-
Progesterone exerts neuroprotective effects after brain injury.Brain Res Rev. 2008 Mar;57(2):386-97. doi: 10.1016/j.brainresrev.2007.06.012. Epub 2007 Jul 27. Brain Res Rev. 2008. PMID: 17826842 Free PMC article. Review.
Cited by
-
Embracing failure: What the Phase III progesterone studies can teach about TBI clinical trials.Brain Inj. 2015;29(11):1259-72. doi: 10.3109/02699052.2015.1065344. Epub 2015 Aug 14. Brain Inj. 2015. PMID: 26274493 Free PMC article. Review.
-
Neuroprotective effects of combined trimetazidine and progesterone on cerebral reperfusion injury.Curr Res Pharmacol Drug Discov. 2022 May 6;3:100108. doi: 10.1016/j.crphar.2022.100108. eCollection 2022. Curr Res Pharmacol Drug Discov. 2022. PMID: 35602337 Free PMC article.
-
Hormonal Changes Associated With Intra-Uterine Growth Restriction: Impact on the Developing Brain and Future Neurodevelopment.Front Endocrinol (Lausanne). 2019 Mar 26;10:179. doi: 10.3389/fendo.2019.00179. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 30972026 Free PMC article. Review.
-
Progesterone inhibition of neuronal calcium signaling underlies aspects of progesterone-mediated neuroprotection.J Steroid Biochem Mol Biol. 2012 Aug;131(1-2):30-6. doi: 10.1016/j.jsbmb.2011.11.002. Epub 2011 Nov 12. J Steroid Biochem Mol Biol. 2012. PMID: 22101209 Free PMC article. Review.
-
The Aging GABAergic System and Its Nutritional Support.J Nutr Metab. 2021 Apr 25;2021:6655064. doi: 10.1155/2021/6655064. eCollection 2021. J Nutr Metab. 2021. PMID: 33986956 Free PMC article. Review.
References
-
- Wright DW, Kellermann AL, Hertzberg VS, Clark PL, Frankel M, Goldstein FC, et al. ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury. Ann Emerg Med. 2007;49:391–402. e1–2. - PubMed
-
- Goss CW, Hoffman SW, Stein DG. Behavioral effects and anatomic correlates after brain injury: a progesterone dose-response study. Pharmacol Biochem Behav. 2003;76:231–42. - PubMed
-
- Robertson CL, Puskar A, Hoffman GE, Murphy AZ, Saraswati M, Fiskum G. Physiologic progesterone reduces mitochondrial dysfunction and hippocampal cell loss after traumatic brain injury in female rats. Exp Neurol. 2006;197:235–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

