Endothelin 1 stimulates Ca2+-sparks and oscillations in retinal arteriolar myocytes via IP3R and RyR-dependent Ca2+ release
- PMID: 21372022
- PMCID: PMC3109063
- DOI: 10.1167/iovs.10-6029
Endothelin 1 stimulates Ca2+-sparks and oscillations in retinal arteriolar myocytes via IP3R and RyR-dependent Ca2+ release
Abstract
Purpose: To investigate endothelin 1 (Et1)-dependent Ca(2+)-signaling at the cellular and subcellular levels in retinal arteriolar myocytes.
Methods: Et1 responses were imaged from Fluo-4-loaded smooth muscle in isolated segments of rat retinal arteriole using confocal laser microscopy.
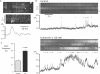
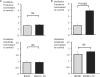
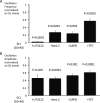
Results: Basal [Ca(2+)](i), subcellular Ca(2+)-sparks, and cellular Ca(2+)-oscillations were all increased during exposure to Et1 (10 nM). Ca(2+)-spark frequency was also increased by 90% by 10 nM Et1. The increase in oscillation frequency was concentration dependent and was inhibited by the EtA receptor (Et(A)R) blocker BQ123 but not by the EtB receptor antagonist BQ788. Stimulation of Ca(2+)-oscillations by Et1 was inhibited by a phospholipase C blocker (U73122; 10 μM), two inhibitors of inositol 1,4,5-trisphosphate receptors (IP(3)Rs), xestospongin C (10 μM), 2-aminoethoxydiphenyl borate (100 μM), and tetracaine (100 μM), a blocker of ryanodine receptors (RyRs).
Conclusions: Et1 stimulates Ca(2+)-sparks and oscillations through Et(A)Rs. The underlying mechanism involves the activation of phospholipase C and both IP(3)Rs and RyRs, suggesting crosstalk between these Ca(2+)-release channels. These findings suggest that phasic Ca(2+)-oscillations play an important role in the smooth muscle response to Et1 within the retinal microvasculature and support an excitatory, proconstrictor role for Ca(2+)-sparks in these vessels.
Figures

Similar articles
-
Feedback via Ca²⁺-activated ion channels modulates endothelin 1 signaling in retinal arteriolar smooth muscle.Invest Ophthalmol Vis Sci. 2012 May 17;53(6):3059-66. doi: 10.1167/iovs.11-9192. Invest Ophthalmol Vis Sci. 2012. PMID: 22427579 Free PMC article.
-
Function and expression of ryanodine receptors and inositol 1,4,5-trisphosphate receptors in smooth muscle cells of murine feed arteries and arterioles.J Physiol. 2012 Apr 15;590(8):1849-69. doi: 10.1113/jphysiol.2011.222083. Epub 2012 Feb 13. J Physiol. 2012. PMID: 22331418 Free PMC article.
-
Two distinct signaling pathways for regulation of spontaneous local Ca2+ release by phospholipase C in airway smooth muscle cells.Pflugers Arch. 2007 Jan;453(4):531-41. doi: 10.1007/s00424-006-0130-1. Epub 2006 Nov 9. Pflugers Arch. 2007. PMID: 17093969
-
Atrial local Ca2+ signaling and inositol 1,4,5-trisphosphate receptors.Prog Biophys Mol Biol. 2010 Sep;103(1):59-70. doi: 10.1016/j.pbiomolbio.2010.02.002. Epub 2010 Mar 1. Prog Biophys Mol Biol. 2010. PMID: 20193706 Review.
-
Interactions between inositol 1,4,5-trisphosphate receptors and ryanodine receptors in smooth muscle: one store or two?Cell Calcium. 2004 Jun;35(6):613-9. doi: 10.1016/j.ceca.2004.01.016. Cell Calcium. 2004. PMID: 15110151 Review.
Cited by
-
Constriction of retinal arterioles to endothelin-1: requisite role of rho kinase independent of protein kinase C and L-type calcium channels.Invest Ophthalmol Vis Sci. 2012 May 17;53(6):2904-12. doi: 10.1167/iovs.12-9542. Invest Ophthalmol Vis Sci. 2012. PMID: 22427601 Free PMC article.
-
Feedback via Ca²⁺-activated ion channels modulates endothelin 1 signaling in retinal arteriolar smooth muscle.Invest Ophthalmol Vis Sci. 2012 May 17;53(6):3059-66. doi: 10.1167/iovs.11-9192. Invest Ophthalmol Vis Sci. 2012. PMID: 22427579 Free PMC article.
-
Function and expression of ryanodine receptors and inositol 1,4,5-trisphosphate receptors in smooth muscle cells of murine feed arteries and arterioles.J Physiol. 2012 Apr 15;590(8):1849-69. doi: 10.1113/jphysiol.2011.222083. Epub 2012 Feb 13. J Physiol. 2012. PMID: 22331418 Free PMC article.
-
Divergent signaling mechanisms for venous versus arterial contraction as revealed by endothelin-1.J Vasc Surg. 2015 Sep;62(3):721-33. doi: 10.1016/j.jvs.2014.03.010. Epub 2014 Apr 14. J Vasc Surg. 2015. PMID: 24726828 Free PMC article.
-
Isolation of Retinal Arterioles for Ex Vivo Cell Physiology Studies.J Vis Exp. 2018 Jul 14;(137):57944. doi: 10.3791/57944. J Vis Exp. 2018. PMID: 30059036 Free PMC article.
References
-
- La M, Reid JJ. Endothelin-1 and the regulation of vascular tone. Clin Exp Pharmacol Physiol. 1995;22:315–323 - PubMed
-
- Jahan H, Kobayashi S, Nishimura J, Kanaide H. Endothelin-1 and angiotensin II act as progression but not competence growth factors in vascular smooth muscle cells. Eur J Pharmacol. 1996;295:261–269 - PubMed
-
- Hafizi S, Allen SP, Goodwin AT, Chester AH, Yacoub MH. Endothelin-1 stimulates proliferation of human coronary smooth muscle cells via the ET(A) receptor and is co-mitogenic with growth factors. Atherosclerosis. 1999;146:351–359 - PubMed
-
- Haynes WG, Webb DJ. Endothelin as a regulator of cardiovascular function in health and disease. J Hypertens. 1998;16:1081–1098 - PubMed
-
- Giaid A, Yanagisawa M, Langleben D, et al. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993;328:1732–1739 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

