Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland
- PMID: 21372033
- PMCID: PMC3071249
- DOI: 10.1158/1940-6207.CAPR-10-0381
Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland
Retraction in
-
Retraction: Obesity Is Associated With Inflammation and Elevated Aromatase Expression in the Mouse Mammary Gland.Cancer Prev Res (Phila). 2022 Jun 2;15(6):413. doi: 10.1158/1940-6207.CAPR-22-0202. Cancer Prev Res (Phila). 2022. PMID: 35652231 Free PMC article. No abstract available.
Abstract
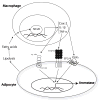
Elevated circulating estrogen levels are associated with increased risk of breast cancer in obese postmenopausal women. Following menopause, the biosynthesis of estrogens through CYP19 (aromatase)-mediated metabolism of androgen precursors occurs primarily in adipose tissue, and the resulting estrogens are then secreted into the systemic circulation. The potential links between obesity, inflammation, and aromatase expression are unknown. In both dietary and genetic models of obesity, we observed necrotic adipocytes surrounded by macrophages forming crown-like structures (CLS) in the mammary glands and visceral fat. The presence of CLS was associated with activation of NF-κB and increased levels of proinflammatory mediators (TNF-α, IL-1β, Cox-2), which were paralleled by elevated levels of aromatase expression and activity in the mammary gland and visceral fat of obese mice. Analyses of the stromal-vascular and adipocyte fractions of the mammary gland suggested that macrophage-derived proinflammatory mediators induced aromatase and estrogen-dependent gene expression (PR, pS2) in adipocytes. Saturated fatty acids, which have been linked to obesity-related inflammation, stimulated NF-κB activity in macrophages leading to increased levels of TNF-α, IL-1β, and Cox-2, each of which contributed to the induction of aromatase in preadipocytes. The discovery of the obesity → inflammation → aromatase axis in the mammary gland and visceral fat and its association with CLS may provide insight into mechanisms underlying the increased risk of hormone receptor-positive breast cancer in obese postmenopausal women, the reduced efficacy of aromatase inhibitors in the treatment of breast cancer in these women, and their generally worse outcomes. The presence of CLS may be a biomarker of increased breast cancer risk or poor prognosis.
Conflict of interest statement
Figures
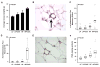
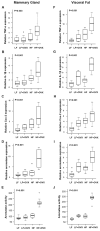

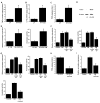
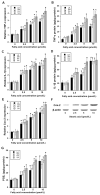
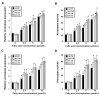
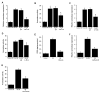
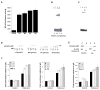
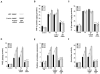
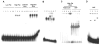
Comment in
-
Inflammatory talk: linking obesity, NF-κB, and Aromatase.Cancer Prev Res (Phila). 2011 Mar;4(3):285-7. doi: 10.1158/1940-6207.CAPR-11-0056. Cancer Prev Res (Phila). 2011. PMID: 21372024
-
Findings of Research Misconduct.Fed Regist. 2023 Sep 13;88(176):62800-62803. Fed Regist. 2023. PMID: 37736072 Free PMC article. No abstract available.
-
Findings of Research Misconduct.Fed Regist. 2023 Sep 13;88(176):62803-62807. Fed Regist. 2023. PMID: 37736073 Free PMC article. No abstract available.
References
-
- Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–91. - PubMed
-
- Santen RJ, Brodie H, Simpson ER, Siiteri PK, Brodie A. History of aromatase: saga of an important biological mediator and therapeutic target. Endocr Rev. 2009;30:343–75. - PubMed
-
- Lorincz AM, Sukumar S. Molecular links between obesity and breast cancer. Endocr Relat Cancer. 2006;13:279–92. - PubMed
-
- Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95:1218–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

