A framework for evaluating biomarkers for early detection: validation of biomarker panels for ovarian cancer
- PMID: 21372037
- PMCID: PMC3057372
- DOI: 10.1158/1940-6207.CAPR-10-0193
A framework for evaluating biomarkers for early detection: validation of biomarker panels for ovarian cancer
Abstract
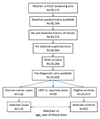
A panel of biomarkers may improve predictive performance over individual markers. Although many biomarker panels have been described for ovarian cancer, few studies used prediagnostic samples to assess the potential of the panels for early detection. We conducted a multisite systematic evaluation of biomarker panels using prediagnostic serum samples from the Prostate, Lung, Colorectal, and Ovarian Cancer (PLCO) screening trial. Using a nested case-control design, levels of 28 biomarkers were measured laboratory-blinded in 118 serum samples obtained before cancer diagnosis and 951 serum samples from matched controls. Five predictive models, each containing 6 to 8 biomarkers, were evaluated according to a predetermined analysis plan. Three sequential analyses were conducted: blinded validation of previously established models (step 1); simultaneous split-sample discovery and validation of models (step 2); and exploratory discovery of new models (step 3). Sensitivity, specificity, sensitivity at 98% specificity, and AUC were computed for the models and CA125 alone among 67 cases diagnosed within one year of blood draw and 476 matched controls. In step 1, one model showed comparable performance to CA125, with sensitivity, specificity, and AUC at 69.2%, 96.6%, and 0.892, respectively. Remaining models had poorer performance than CA125 alone. In step 2, we observed a similar pattern. In step 3, a model derived from all 28 markers failed to show improvement over CA125. Thus, biomarker panels discovered in diagnostic samples may not validate in prediagnostic samples; utilizing prediagnostic samples for discovery may be helpful in developing validated early detection panels.
Figures

Comment in
-
The sine qua non of discovering novel biomarkers for early detection of ovarian cancer: carefully selected preclinical samples.Cancer Prev Res (Phila). 2011 Mar;4(3):299-302. doi: 10.1158/1940-6207.CAPR-11-0048. Cancer Prev Res (Phila). 2011. PMID: 21372028
-
Challenges related to developing serum-based biomarkers for early ovarian cancer detection.Cancer Prev Res (Phila). 2011 Mar;4(3):303-6. doi: 10.1158/1940-6207.CAPR-11-0053. Cancer Prev Res (Phila). 2011. PMID: 21372029 Free PMC article.
References
-
- Petricoin EF, Ardekani AM, Hitt BA, et al. Use of proteomic patterns in serum to identify ovarian cancer. Lancet. 2002;359:572–577. - PubMed
-
- Zhang Z, Bast RC, Jr, Yu Y, et al. Three biomarkers identified from serum proteomic analysis for the detection of early stage ovarian cancer. Cancer Res. 2004;64:5882–5890. - PubMed
-
- Gorelik E, Landsittel DP, Marrangoni AM, et al. Multiplexed Immunobead-Based Cytokine Profiling for Early Detection of Ovarian Cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:981–987. - PubMed
-
- Visintin I, Feng Z, Longton G, et al. Diagnostic markers for early detection of ovarian cancer. Clin Cancer Res. 2008;14:1065–1072. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

