Ethanol enhances carbachol-induced protease activation and accelerates Ca2+ waves in isolated rat pancreatic acini
- PMID: 21372126
- PMCID: PMC3077610
- DOI: 10.1074/jbc.M110.196832
Ethanol enhances carbachol-induced protease activation and accelerates Ca2+ waves in isolated rat pancreatic acini
Abstract
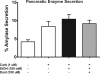
Alcohol abuse is a leading cause of pancreatitis, accounting for 30% of acute cases and 70-90% of chronic cases, yet the mechanisms leading to alcohol-associated pancreatic injury are unclear. An early and critical feature of pancreatitis is the aberrant signaling of Ca(2+) within the pancreatic acinar cell. An important conductor of this Ca(2+) is the basolaterally localized, intracellular Ca(2+) channel ryanodine receptor (RYR). In this study, we examined the effect of ethanol on mediating both pathologic intra-acinar protease activation, a precursor to pancreatitis, as well as RYR Ca(2+) signals. We hypothesized that ethanol sensitizes the acinar cell to protease activation by modulating RYR Ca(2+). Acinar cells were freshly isolated from rat, pretreated with ethanol, and stimulated with the muscarinic agonist carbachol (1 μM). Ethanol caused a doubling in the carbachol-induced activation of the proteases trypsin and chymotrypsin (p < 0.02). The RYR inhibitor dantrolene abrogated the enhancement of trypsin and chymotrypsin activity by ethanol (p < 0.005 for both proteases). Further, ethanol accelerated the speed of the apical to basolateral Ca(2+) wave from 9 to 18 μm/s (p < 0.0005; n = 18-22 cells/group); an increase in Ca(2+) wave speed was also observed with a change from physiologic concentrations of carbachol (1 μM) to a supraphysiologic concentration (1 mM) that leads to protease activation. Dantrolene abrogated the ethanol-induced acceleration of wave speed (p < 0.05; n = 10-16 cells/group). Our results suggest that the enhancement of pathologic protease activation by ethanol is dependent on the RYR and that a novel mechanism for this enhancement may involve RYR-mediated acceleration of Ca(2+) waves.
Figures








References
-
- Lowenfels A. B., Sullivan T., Fiorianti J., Maisonneuve P. (2005) Curr. Gastroenterol. Rep. 7, 90–95 - PubMed
-
- Steinberg W., Tenner S. (1994) N. Engl. J. Med. 330, 1198–1210 - PubMed
-
- Haber P. S., Wilson J. S., Apte M. V., Pirola R. C. (1993) J. Lab. Clin. Med. 121, 759–764 - PubMed
-
- Criddle D. N., Murphy J., Fistetto G., Barrow S., Tepikin A. V., Neoptolemos J. P., Sutton R., Petersen O. H. (2006) Gastroenterology 130, 781–793 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

