Addition of atrasentan to renin-angiotensin system blockade reduces albuminuria in diabetic nephropathy
- PMID: 21372210
- PMCID: PMC3065231
- DOI: 10.1681/ASN.2010080869
Addition of atrasentan to renin-angiotensin system blockade reduces albuminuria in diabetic nephropathy
Abstract
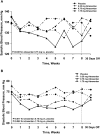
Although endothelin-receptor antagonists reduce albuminuria in diabetic nephropathy, fluid retention limits their use. Here, we examined the effect of atrasentan, a selective endothelin A receptor (ET(A)R) antagonist, on albuminuria in a randomized, double-blind, placebo-controlled trial of subjects with diabetic nephropathy already receiving stable doses of renin-angiotensin system (RAS) inhibitors. We randomly assigned 89 subjects with eGFR >20 ml/min per 1.73 m(2) and a urinary albumin-to-creatinine ratio (UACR) of 100 to 3000 mg/g to placebo or atrasentan (0.25, 0.75, or 1.75 mg daily) for 8 weeks. Compared with placebo, atrasentan significantly reduced UACR only in the 0.75- and 1.75-mg groups (P=0.001 and P=0.011, respectively). Compared with the 11% reduction in the geometric mean of the UACR from baseline to final observation in the placebo group during the study, the geometric mean of UACR decreased by 21, 42, and 35% in the 0.25-, 0.75-, and 1.75-mg atrasentan groups (P=0.291, P=0.023, and P=0.073, respectively). In the placebo group, 17% of subjects achieved ≥40% reduction in UACR from baseline compared with 30, 50, and 38% in the 0.25-, 0.75-, and 1.75-mg atrasentan groups, respectively (P=0.029 for 0.75 mg versus placebo). Peripheral edema occurred in 9% of subjects receiving placebo and in 14, 18, and 46% of those receiving 0.25, 0.5, and 1.75 mg atrasentan, respectively (P=0.007 for 1.75 mg versus placebo). In summary, atrasentan, at the doses tested, is generally safe and effective in reducing residual albuminuria and may ultimately improve renal outcomes in patients with type 2 diabetic nephropathy.
Copyright © 2011 by the American Society of Nephrology
Figures

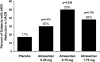

Comment in
-
Endothelin antagonist as add-on treatment for proteinuria in diabetic nephropathy: is there light at the end of the tunnel?J Am Soc Nephrol. 2011 Apr;22(4):593-5. doi: 10.1681/ASN.2011020158. Epub 2011 Mar 17. J Am Soc Nephrol. 2011. PMID: 21415160 No abstract available.
References
-
- Gaede P, Lund-Andersen H, Parving HH, Pedersen O: Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 358: 580–591, 2008 - PubMed
-
- Gilbertson DT, Liu J, Xue JL, Louis TA, Solid CA, Ebben JP, Collins AJ: Projecting the number of patients with end-stage renal disease in the United States to the year 2015. J Am Soc Nephrol 16: 3736–3741, 2005 - PubMed
-
- Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S: Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 - PubMed
-
- Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I: Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345: 851–860, 2001 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

