Oxidative stress resistance in Deinococcus radiodurans
- PMID: 21372322
- PMCID: PMC3063356
- DOI: 10.1128/MMBR.00015-10
Oxidative stress resistance in Deinococcus radiodurans
Abstract
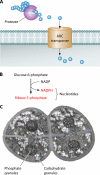
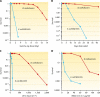
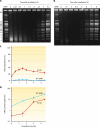
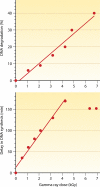
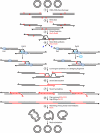
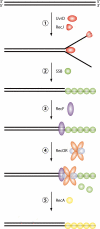
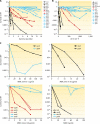
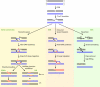
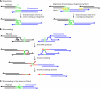
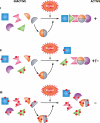
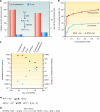
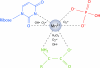
Deinococcus radiodurans is a robust bacterium best known for its capacity to repair massive DNA damage efficiently and accurately. It is extremely resistant to many DNA-damaging agents, including ionizing radiation and UV radiation (100 to 295 nm), desiccation, and mitomycin C, which induce oxidative damage not only to DNA but also to all cellular macromolecules via the production of reactive oxygen species. The extreme resilience of D. radiodurans to oxidative stress is imparted synergistically by an efficient protection of proteins against oxidative stress and an efficient DNA repair mechanism, enhanced by functional redundancies in both systems. D. radiodurans assets for the prevention of and recovery from oxidative stress are extensively reviewed here. Radiation- and desiccation-resistant bacteria such as D. radiodurans have substantially lower protein oxidation levels than do sensitive bacteria but have similar yields of DNA double-strand breaks. These findings challenge the concept of DNA as the primary target of radiation toxicity while advancing protein damage, and the protection of proteins against oxidative damage, as a new paradigm of radiation toxicity and survival. The protection of DNA repair and other proteins against oxidative damage is imparted by enzymatic and nonenzymatic antioxidant defense systems dominated by divalent manganese complexes. Given that oxidative stress caused by the accumulation of reactive oxygen species is associated with aging and cancer, a comprehensive outlook on D. radiodurans strategies of combating oxidative stress may open new avenues for antiaging and anticancer treatments. The study of the antioxidation protection in D. radiodurans is therefore of considerable potential interest for medicine and public health.
Figures


References
-
- Abed, R. M., B. Zein, A. Al-Thukair, and D. de Beer. 2007. Phylogenetic diversity and activity of aerobic heterotrophic bacteria from a hypersaline oil-polluted microbial mat. Syst. Appl. Microbiol. 30:319-330. - PubMed
-
- Reference deleted.
-
- Abreu, I. A., et al. 2008. The kinetic mechanism of manganese-containing superoxide dismutase from Deinococcus radiodurans: a specialized enzyme for the elimination of high superoxide concentrations. Biochemistry 47:2350-2356. - PubMed
-
- Aguilaniu, H., L. Gustafsson, M. Rigoulet, and T. Nystrom. 2001. Protein oxidation in G0 cells of Saccharomyces cerevisiae depends on the state rather than rate of respiration and is enhanced in pos9 but not yap1 mutants. J. Biol. Chem. 276:35396-35404. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

