Analysis of protein-ligand interactions by fluorescence polarization
- PMID: 21372817
- PMCID: PMC3160472
- DOI: 10.1038/nprot.2011.305
Analysis of protein-ligand interactions by fluorescence polarization
Abstract
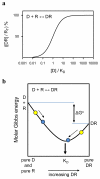
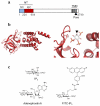
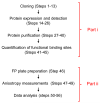
Quantification of the associations between biomolecules is required both to predict and understand the interactions that underpin all biological activity. Fluorescence polarization (FP) provides a nondisruptive means of measuring the association of a fluorescent ligand with a larger molecule. We describe an FP assay in which binding of fluorescein-labeled inositol 1,4,5-trisphosphate (IP(3)) to N-terminal fragments of IP(3) receptors can be characterized at different temperatures and in competition with other ligands. The assay allows the standard Gibbs free energy (ΔG°), enthalpy (ΔH°) and entropy (ΔS°) changes of ligand binding to be determined. The method is applicable to any purified ligand-binding site for which an appropriate fluorescent ligand is available. FP can be used to measure low-affinity interactions in real time without the use of radioactive materials, it is nondestructive and, with appropriate care, it can resolve ΔH° and ΔS°. The first part of the protocol, protein preparation, may take several weeks, whereas the FP measurements, once they have been optimized, would normally take 1-6 h.
Figures




References
-
- Foreman JC, Johansen T, editors. Textbook of receptor pharmacology. 2nd ed CRC Press; Boca Raton, Florida: 2003.
-
- Kenakin TP. A pharmacology primer. Theory, application, and methods. Elsevier; San Diego: 2004.
-
- Wyman J, Gill SJ. Binding and linkage. Functional chemistry of biological macromolecules. University Science Books; Mill Valley, California: 1990.
-
- Keeler J, Wothers P. Chemical structure and reactivity. An integrated approach. Oxford University Press; Oxford: 2008.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

