Dynamic clamp with StdpC software
- PMID: 21372819
- PMCID: PMC3188375
- DOI: 10.1038/nprot.2010.200
Dynamic clamp with StdpC software
Abstract
Dynamic clamp is a powerful method that allows the introduction of artificial electrical components into target cells to simulate ionic conductances and synaptic inputs. This method is based on a fast cycle of measuring the membrane potential of a cell, calculating the current of a desired simulated component using an appropriate model and injecting this current into the cell. Here we present a dynamic clamp protocol using free, fully integrated, open-source software (StdpC, for spike timing-dependent plasticity clamp). Use of this protocol does not require specialist hardware, costly commercial software, experience in real-time operating systems or a strong programming background. The software enables the configuration and operation of a wide range of complex and fully automated dynamic clamp experiments through an intuitive and powerful interface with a minimal initial lead time of a few hours. After initial configuration, experimental results can be generated within minutes of establishing cell recording.
Figures

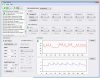
General menus for file control (loading/saving protocols and scripts) and configuration.
Start and Stop button to control execution of the dynamic clamp
Dropdown choice of the used hardware driver
Message window displaying status information and a log of user actions
Panel for configuring of up to 6 different simulated synapses
Panel for configuring up to 6 ionic conductances
Spike generator module for configuring computer generated neuronal activity.
Data displays for debugging the dynamic clamp configuration.
References
-
- Robinson HP, Kawai N. Injection of digitally synthesized synaptic conductance transients to measure the integrative properties of neurons. J. Neurosci. Methods. 1993;49(3):157–165. - PubMed
-
- Sharp AA, O’Neil MB, Abbott LF, Marder E. The dynamic clamp: artificial conductances in biological neurons. Trends Neurosci. 1993;16(10):389–394. - PubMed
-
- Sharp AA, Skinner FK, Marder E. Mechanisms of oscillation in dynamic clamp constructed two-cell half-center circuits. J. Neurophysiol. 1996;76:867–883. - PubMed
-
- Elson RC, Selverston AI, Abarbanel HDI, Rabinovich MI. Inhibitory synchronization of bursting in biological neurons: dependence on synaptic time constant. J. Neurophysiol. 2002;88:1166–1176. - PubMed
-
- Szücs A, Elson RC, Rabinovich MI, Abarbanel HDI, Selverston AI. Nonlinear behavior of sinusoidally forced pyloric pacemaker neurons. J. Neurophysiol. 2001;85(4):1623–38. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

