Reduced metabolism in brain "control networks" following cocaine-cues exposure in female cocaine abusers
- PMID: 21373180
- PMCID: PMC3043072
- DOI: 10.1371/journal.pone.0016573
Reduced metabolism in brain "control networks" following cocaine-cues exposure in female cocaine abusers
Abstract
Objective: Gender differences in vulnerability for cocaine addiction have been reported. Though the mechanisms are not understood, here we hypothesize that gender differences in reactivity to conditioned-cues, which contributes to relapse, are involved.
Method: To test this we compared brain metabolism (using PET and ¹⁸FDG) between female (n = 10) and male (n = 16) active cocaine abusers when they watched a neutral video (nature scenes) versus a cocaine-cues video.
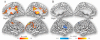
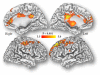
Results: Self-reports of craving increased with the cocaine-cue video but responses did not differ between genders. In contrast, changes in whole brain metabolism with cocaine-cues differed by gender (p<0.05); females significantly decreased metabolism (-8.6%±10) whereas males tended to increase it (+5.5%±18). SPM analysis (Cocaine-cues vs Neutral) in females revealed decreases in frontal, cingulate and parietal cortices, thalamus and midbrain (p<0.001) whereas males showed increases in right inferior frontal gyrus (BA 44/45) (only at p<0.005). The gender-cue interaction showed greater decrements with Cocaine-cues in females than males (p<0.001) in frontal (BA 8, 9, 10), anterior cingulate (BA 24, 32), posterior cingulate (BA 23, 31), inferior parietal (BA 40) and thalamus (dorsomedial nucleus).
Conclusions: Females showed greater brain reactivity to cocaine-cues than males but no differences in craving, suggesting that there may be gender differences in response to cues that are not linked with craving but could affect subsequent drug use. Specifically deactivation of brain regions from "control networks" (prefrontal, cingulate, inferior parietal, thalamus) in females could increase their vulnerability to relapse since it would interfere with executive function (cognitive inhibition). This highlights the importance of gender tailored interventions for cocaine addiction.
Conflict of interest statement
Figures
Similar articles
-
Methylphenidate attenuates limbic brain inhibition after cocaine-cues exposure in cocaine abusers.PLoS One. 2010 Jul 9;5(7):e11509. doi: 10.1371/journal.pone.0011509. PLoS One. 2010. PMID: 20634975 Free PMC article. Clinical Trial.
-
Cognitive control of drug craving inhibits brain reward regions in cocaine abusers.Neuroimage. 2010 Feb 1;49(3):2536-43. doi: 10.1016/j.neuroimage.2009.10.088. Epub 2009 Nov 11. Neuroimage. 2010. PMID: 19913102 Free PMC article.
-
Association of methylphenidate-induced craving with changes in right striato-orbitofrontal metabolism in cocaine abusers: implications in addiction.Am J Psychiatry. 1999 Jan;156(1):19-26. doi: 10.1176/ajp.156.1.19. Am J Psychiatry. 1999. PMID: 9892293
-
Synaptic mechanisms underlying persistent cocaine craving.Nat Rev Neurosci. 2016 Jun;17(6):351-65. doi: 10.1038/nrn.2016.39. Epub 2016 May 6. Nat Rev Neurosci. 2016. PMID: 27150400 Free PMC article. Review.
-
Serotonin at the nexus of impulsivity and cue reactivity in cocaine addiction.Neuropharmacology. 2014 Jan;76 Pt B(0 0):460-78. doi: 10.1016/j.neuropharm.2013.06.030. Epub 2013 Jul 11. Neuropharmacology. 2014. PMID: 23850573 Free PMC article. Review.
Cited by
-
The development of a preference for cocaine over food identifies individual rats with addiction-like behaviors.PLoS One. 2013 Nov 18;8(11):e79465. doi: 10.1371/journal.pone.0079465. eCollection 2013. PLoS One. 2013. PMID: 24260227 Free PMC article.
-
Cannabis Abusers Show Hypofrontality and Blunted Brain Responses to a Stimulant Challenge in Females but not in Males.Neuropsychopharmacology. 2016 Sep;41(10):2596-605. doi: 10.1038/npp.2016.67. Epub 2016 May 9. Neuropsychopharmacology. 2016. PMID: 27156854 Free PMC article.
-
Neuroimmunometabolism: A New Pathological Nexus Underlying Neurodegenerative Disorders.J Neurosci. 2022 Mar 9;42(10):1888-1907. doi: 10.1523/JNEUROSCI.0998-21.2022. Epub 2022 Jan 13. J Neurosci. 2022. PMID: 35027409 Free PMC article. Review.
-
Cocaine differentially affects synaptic activity in memory and midbrain areas of female and male rats: an in vivo MEMRI study.Brain Imaging Behav. 2018 Feb;12(1):201-216. doi: 10.1007/s11682-017-9691-1. Brain Imaging Behav. 2018. PMID: 28236167 Free PMC article.
-
Striatocortical pathway dysfunction in addiction and obesity: differences and similarities.Crit Rev Biochem Mol Biol. 2013 Jan-Feb;48(1):1-19. doi: 10.3109/10409238.2012.735642. Epub 2012 Nov 23. Crit Rev Biochem Mol Biol. 2013. PMID: 23173916 Free PMC article. Review.
References
-
- Evans SM. The role of estradiol and progesterone in modulating the subjective effects of stimulants in humans. Exp Clin Psychopharmacol. 2007;15(5):418–426. - PubMed
-
- Zilberman M, Tavares H, el-Guebaly N. Gender similarities and differences: the prevalence and course of alcohol- and other substance-related disorders. J Addict Dis. 2003;22(4):61–74. - PubMed
-
- Hernandez-Avila CA, Rounsaville BJ, Kranzler HR. Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend. 2004;74(3):265–272. - PubMed
-
- O'Brien MS, Anthony JC. Risk of becoming cocaine dependent: Epidemiological estimates for the United States, 2000–2001. Neuropsychopharmacology. 2005;30:1006–1018. - PubMed
-
- Anglin MD, Hser YI, McGlothlin WH. Sex differences in addict careers. 2. Becoming addicted. Am J Drug Alcohol Abuse. 1987;13(1–2):59–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

