Adverse event assessment of antimuscarinics for treating overactive bladder: a network meta-analytic approach
- PMID: 21373193
- PMCID: PMC3044140
- DOI: 10.1371/journal.pone.0016718
Adverse event assessment of antimuscarinics for treating overactive bladder: a network meta-analytic approach
Abstract
Background: Overactive bladder (OAB) affects the lives of millions of people worldwide and antimuscarinics are the pharmacological treatment of choice. Meta-analyses of all currently used antimuscarinics for treating OAB found similar efficacy, making the choice dependent on their adverse event profiles. However, conventional meta-analyses often fail to quantify and compare adverse events across different drugs, dosages, formulations, and routes of administration. In addition, the assessment of the broad variety of adverse events is dissatisfying. Our aim was to compare adverse events of antimuscarinics using a network meta-analytic approach that overcomes shortcomings of conventional analyses.
Methods: Cochrane Incontinence Group Specialized Trials Register, previous systematic reviews, conference abstracts, book chapters, and reference lists of relevant articles were searched. Eligible studies included randomized controlled trials comparing at least one antimuscarinic for treating OAB with placebo or with another antimuscarinic, and adverse events as outcome measures. Two authors independently extracted data. A network meta-analytic approach was applied allowing for joint assessment of all adverse events of all currently used antimuscarinics while fully maintaining randomization.
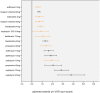
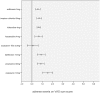
Results: 69 trials enrolling 26'229 patients were included. Similar overall adverse event profiles were found for darifenacin, fesoterodine, transdermal oxybutynin, propiverine, solifenacin, tolterodine, and trospium chloride but not for oxybutynin orally administered when currently used starting dosages were compared.
Conclusions: The proposed generally applicable transparent network meta-analytic approach summarizes adverse events in an easy to grasp way allowing straightforward benchmarking of antimuscarinics for treating OAB in clinical practice. Most currently used antimuscarinics seem to be equivalent first choice drugs to start the treatment of OAB except for oral oxybutynin dosages of ≥ 10 mg/d which may have more unfavorable adverse event profiles.
Conflict of interest statement
Figures
References
-
- Milsom I, Abrams P, Cardozo L, Roberts RG, Thüroff J, et al. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int. 2001;87:760–766. - PubMed
-
- Abrams P, Kelleher CJ, Kerr LA, Rogers RG. Overactive bladder significantly affects quality of life. Am J Manag Care. 2000;6:S580–590. - PubMed
-
- Stewart WF, Van Rooyen JB, Cundiff GW, Abrams P, Herzog AR, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20:327–336. - PubMed
-
- Coyne KS, Margolis MK, Jumadilova Z, Bavendam T, Mueller E, et al. Overactive bladder and women's sexual health: what is the impact? J Sex Med. 2007;4:656–666. - PubMed
-
- Brown JS, Vittinghoff E, Wyman JF, Stone KL, Nevitt MC, et al. Urinary incontinence: does it increase risk for falls and fractures? Study of Osteoporotic Fractures Research Group. J Am Geriatr Soc. 2000;48:721–725. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

