Efficacy of CMX001 as a post exposure antiviral in New Zealand White rabbits infected with rabbitpox virus, a model for orthopoxvirus infections of humans
- PMID: 21373379
- PMCID: PMC3046869
- DOI: 10.3390/v3010047
Efficacy of CMX001 as a post exposure antiviral in New Zealand White rabbits infected with rabbitpox virus, a model for orthopoxvirus infections of humans
Abstract
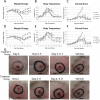
CMX001, a lipophilic nucleotide analog formed by covalently linking 3-(hexdecyloxy)propan-1-ol to cidofovir (CDV), is being developed as a treatment for smallpox. In the absence of human cases of smallpox, new treatments must be tested for efficacy in animal models. Previously, we demonstrated the efficacy of CMX001 in protecting New Zealand White rabbits from mortality following intradermal infection with rabbitpox virus as a model for smallpox, monkeypox and for treatment of adverse reactions to smallpox vaccination. Here we extend these studies by exploring different dosing regimens and performing randomized, blinded, placebo-controlled studies. In addition, because rabbitpox virus can be transmitted via naturally generated aerosols (animal to animal transmission), we report on studies to test the efficacy of CMX001 in protecting rabbits from lethal rabbitpox virus disease when infection occurs by animal to animal transmission. In all cases, CMX001 treatment was initiated at the onset of observable lesions in the ears to model the use of CMX001 as a treatment for symptomatic smallpox. The results demonstrate that CMX001 is an effective treatment for symptomatic rabbitpox virus infection. The rabbitpox model has key similarities to human smallpox including an incubation period, generalized systemic disease, the occurrence of lesions which may be used as a trigger for initiating therapy, and natural animal to animal spread, making it an appropriate model.
Keywords: CMX001; antiviral; poxvirus; rabbitpox; smallpox treatment.
Figures
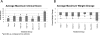





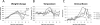
References
-
- FDA grants marketing clearance of Vistide for the treatment of CMV retinitis. AIDS Patient Care STDS. 1996;10:383–384. - PubMed
-
- Food and Drug Administration. FDA approves cidofovir for treatment of CMV retinitis. J Int Assoc Phys AIDS Care. 1996;2:30. - PubMed
-
- Bray M, Martinez M, Smee DF, Kefauver D, Thompson E, Huggins JW. Cidofovir protects mice against lethal aerosol or intranasal cowpox virus challenge. J Infect Dis. 2000;181:10–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
