Membrane cholesterol modulates cochlear electromechanics
- PMID: 21373862
- PMCID: PMC3098987
- DOI: 10.1007/s00424-011-0942-5
Membrane cholesterol modulates cochlear electromechanics
Abstract
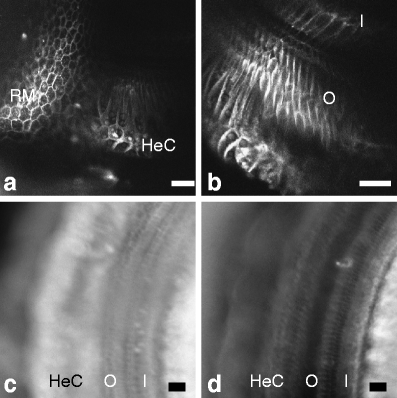
Changing the concentration of cholesterol in the plasma membrane of isolated outer hair cells modulates electromotility and prestin-associated charge movement, suggesting that a similar manipulation would alter cochlear mechanics. We examined cochlear function before and after depletion of membrane cholesterol with methyl-β-cyclodextrin (MβCD) in an excised guinea pig temporal bone preparation. The mechanical response of the cochlear partition to acoustic and/or electrical stimulation was monitored using laser interferometry and time-resolved confocal microscopy. The electromechanical response in untreated preparations was asymmetric with greater displacements in response to positive currents. Exposure to MβCD increased the magnitude and asymmetry of the response, without changing the frequency tuning of sound-evoked mechanical responses or cochlear microphonic potentials. Sodium salicylate reversibly blocked the enhanced electromechanical response in cholesterol depleted preparations. The increase of sound-evoked vibrations during positive current injection was enhanced following MβCD in some preparations. Imaging was used to assess cellular integrity which remained unchanged after several hours of exposure to MβCD in several preparations. The enhanced electromechanical response reflects an increase in outer hair cell electromotility and may reveal features of cholesterol distribution and trafficking in outer hair cells.
Figures

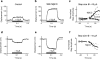



References
-
- Alberts B. Molecular biology of the cell. 4. New York: Garland Science; 2002.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

