Loss of one Tgfbr2 allele in fibroblasts promotes metastasis in MMTV: polyoma middle T transgenic and transplant mouse models of mammary tumor progression
- PMID: 21374085
- PMCID: PMC3373018
- DOI: 10.1007/s10585-011-9373-0
Loss of one Tgfbr2 allele in fibroblasts promotes metastasis in MMTV: polyoma middle T transgenic and transplant mouse models of mammary tumor progression
Abstract
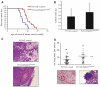
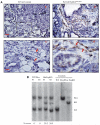
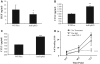
Accumulation of fibroblasts is a phenomenon that significantly correlates with formation of aggressive cancers. While studies have shown that the TGF-β signaling pathway is an important regulator of fibroblast activation, the functional contribution of TGF-β signaling in fibroblasts during multi-step tumor progression remains largely unclear. In previous studies, we used a sub-renal capsule transplantation model to demonstrate that homozygous knockout of the Tgfbr2 gene (Tgbr2(FspKO)) enhanced mammary tumor growth and metastasis. Here, we show for the first time a significant role for loss of one Tgfbr2 allele during multi-step mammary tumor progression. Heterozygous deletion of Tgfbr2 in stromal cells in MMTV-PyVmT transgenic mice (PyVmT/Tgfbr2(hetFspKO) mice) resulted in earlier tumor formation and increased stromal cell accumulation. In contrast to previous studies of Tgbr2(FspKO) fibroblasts, Tgfbr2(hetFspKO) fibroblasts did not significantly increase tumor growth, but enhanced lung metastasis in PyVmT transgenic mice and in co-transplantation studies with PyVmT mammary carcinoma cells. Furthermore, Tgfbr2(hetFspKO) fibroblasts enhanced mammary carcinoma cell invasiveness associated with expression of inflammatory cytokines including CXCL12 and CCL2. Analyses of Tgbr2(FspKO) and Tgfbr2(hetFspKO) fibroblasts revealed differences in the expression of factors associated with metastatic spread, indicating potential differences in the mechanism of action between homozygous and heterozygous deletion of Tgfbr2 in stromal cells. In summary, these studies demonstrate for the first time that loss of one Tgfbr2 allele in fibroblasts enhances mammary metastases in a multi-step model of tumor progression, and demonstrate the importance of clarifying the functional contribution of genetic alterations in stromal cells in breast cancer progression.
Figures




References
-
- Desmouliere A, Guyot C, Gabbiani G. The stroma reaction myofibroblast: a key player in the control of tumor cell behavior. Int J Dev Biol. 2004;48(5-6):509–517. - PubMed
-
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6(5):392–401. - PubMed
-
- Sakakura T, Sakagami Y, Nishizuka Y. Accelerated mammary cancer development by fetal salivary mesenchyma isografted to adult mouse mammary epithelium. J Natl Cancer Inst. 1981;66(5):953–959. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

