Structure, signaling mechanism and regulation of the natriuretic peptide receptor guanylate cyclase
- PMID: 21375693
- PMCID: PMC3097287
- DOI: 10.1111/j.1742-4658.2011.08083.x
Structure, signaling mechanism and regulation of the natriuretic peptide receptor guanylate cyclase
Abstract
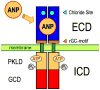
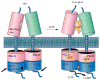
Atrial natriuretic peptide (ANP) and the homologous B-type natriuretic peptide are cardiac hormones that dilate blood vessels and stimulate natriuresis and diuresis, thereby lowering blood pressure and blood volume. ANP and B-type natriuretic peptide counterbalance the actions of the renin-angiotensin-aldosterone and neurohormonal systems, and play a central role in cardiovascular regulation. These activities are mediated by natriuretic peptide receptor-A (NPRA), a single transmembrane segment, guanylyl cyclase (GC)-linked receptor that occurs as a homodimer. Here, we present an overview of the structure, possible chloride-mediated regulation and signaling mechanism of NPRA and other receptor GCs. Earlier, we determined the crystal structures of the NPRA extracellular domain with and without bound ANP. Their structural comparison has revealed a novel ANP-induced rotation mechanism occurring in the juxtamembrane region that apparently triggers transmembrane signal transduction. More recently, the crystal structures of the dimerized catalytic domain of green algae GC Cyg12 and that of cyanobacterium GC Cya2 have been reported. These structures closely resemble that of the adenylyl cyclase catalytic domain, consisting of a C1 and C2 subdomain heterodimer. Adenylyl cyclase is activated by binding of G(s)α to C2 and the ensuing 7° rotation of C1 around an axis parallel to the central cleft, thereby inducing the heterodimer to adopt a catalytically active conformation. We speculate that, in NPRA, the ANP-induced rotation of the juxtamembrane domains, transmitted across the transmembrane helices, may induce a similar rotation in each of the dimerized GC catalytic domains, leading to the stimulation of the GC catalytic activity.
© 2011 The Authors Journal compilation © 2011 FEBS.
Figures



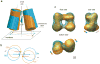
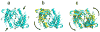
References
-
- de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Science. 1981;28:89–94. - PubMed
-
- Currie MG, Geller DM, Cole BR, Boylan JG, YuSheng W, Holmberg SW, Needleman P. Bioactive cardiac substances: potent vasorelaxant activity in mammalian atria. Science. 1983;221:71–73. - PubMed
-
- Grammer RT, Fukumi H, Inagami T, Misono KS. Rat atrial natriuretic factor. Purification and vasorelaxant activity. Biochem Biophys Res Commun. 1983;116:696–703. - PubMed
-
- Lopez MJ, Wong SK, Kishimoto I, Dubois S, Mach V, Friesen J, Garbers DL, Beuve A. Salt-resistant hypertension in mice lacking the guanylyl cyclase-A receptor for atrial natriuretic peptide. Nature. 1995;378:65–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

