Constitutive Gs activation using a single-construct tetracycline-inducible expression system in embryonic stem cells and mice
- PMID: 21375737
- PMCID: PMC3226282
- DOI: 10.1186/scrt52
Constitutive Gs activation using a single-construct tetracycline-inducible expression system in embryonic stem cells and mice
Abstract
Introduction: The controlled expression of many genes, including G-protein coupled receptors (GPCRs), is important for delineating gene functions in complex model systems. Binary systems for inducible regulation of transgene expression are widely used in mice. One system is the tTA/TRE expression system, composed of a tetracycline-dependent DNA binding factor and a separate tetracycline operon. However, the requirement for two separate transgenes (one for each tTA or TRE component) makes this system less amenable to models requiring directed cell targeting, increases the risk of multiple transgene integration sites, and requires extensive screening for appropriately-functioning clones.
Methods: We developed a single, polycistronic tetracycline-inducible expression platform to control the expression of multiple cistrons in mammalian cells. This platform has three basic constructs: regulator, responder, and destination vectors. The modular platform is compatible with both the TetOff (tTA) and TetOn (rtTA) systems. The modular Gateway recombineering-compatible components facilitate rapidly generating vectors to genetically modify mammalian cells. We apply this system to use the elongation factor 1α (EF1α) promoter to drive doxycycline-regulated expression of both the fluorescent marker mCherry and an engineered Gs-coupled GPCR "Rs1" separated by a 2A ribosomal skip site.
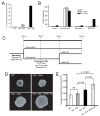
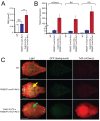
Results: We show that our combined expression construct drives expression of both the mCherry and Rs1 transgenes in a doxycycline-dependent manner. We successfully target the expression construct into the Rosa26 locus of mouse embryonic stem (ES) cells. Rs1 expression in mouse ES cells increases cAMP accumulation via both basal and ligand-induced Gs mechanisms and is associated with increased embryoid body size. Heterozygous mice carrying the Rs1 expression construct showed normal growth and weight, and developed small increases in bone formation that could be observed in the calvaria.
Conclusions: Our results demonstrate the feasibility of a single-vector strategy that combines both the tTA and TRE tetracycline-regulated components for use in cells and mouse models. Although the EF1α promoter is useful for driving expression in pluripotent cells, a single copy of the EF1α promoter did not drive high levels of mCherry and Rs1 expression in the differentiated tissues of adult mice. These findings indicate that promoter selection is an important factor when developing transgene expression models.
Figures

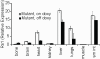
Similar articles
-
Targeted expression of two proteins in neural retina using self-inactivating, insulated lentiviral vectors carrying two internal independent promoters.Mol Vis. 2007 Oct 18;13:2001-11. Mol Vis. 2007. PMID: 17982424
-
Generation and Characterization of Inducible Lung and Skin-Specific IL-22 Transgenic Mice.Methods Mol Biol. 2021;2223:115-132. doi: 10.1007/978-1-0716-1001-5_9. Methods Mol Biol. 2021. PMID: 33226591
-
Targeted transgenesis at the HPRT locus: an efficient strategy to achieve tightly controlled in vivo conditional expression with the tet system.Physiol Genomics. 2009 Apr 10;37(2):140-6. doi: 10.1152/physiolgenomics.90328.2008. Epub 2009 Jan 13. Physiol Genomics. 2009. PMID: 19141541
-
In vivo gene regulation using tetracycline-regulatable systems.Adv Drug Deliv Rev. 2009 Jul 2;61(7-8):527-41. doi: 10.1016/j.addr.2008.12.016. Epub 2009 Apr 23. Adv Drug Deliv Rev. 2009. PMID: 19394373 Free PMC article. Review.
-
Genetic mouse models for bone studies--strengths and limitations.Bone. 2011 Dec;49(6):1242-54. doi: 10.1016/j.bone.2011.08.021. Epub 2011 Aug 31. Bone. 2011. PMID: 21907838 Free PMC article. Review.
Cited by
-
WNT6 is a novel oncogenic prognostic biomarker in human glioblastoma.Theranostics. 2018 Sep 9;8(17):4805-4823. doi: 10.7150/thno.25025. eCollection 2018. Theranostics. 2018. PMID: 30279739 Free PMC article.
-
Controlled re-activation of epigenetically silenced Tet promoter-driven transgene expression by targeted demethylation.Nucleic Acids Res. 2017 Sep 19;45(16):e147. doi: 10.1093/nar/gkx601. Nucleic Acids Res. 2017. PMID: 28934472 Free PMC article.
-
Acute Vhl gene inactivation induces cardiac HIF-dependent erythropoietin gene expression.PLoS One. 2011;6(7):e22589. doi: 10.1371/journal.pone.0022589. Epub 2011 Jul 21. PLoS One. 2011. PMID: 21811636 Free PMC article.
-
Targeted disruption of DNMT1, DNMT3A and DNMT3B in human embryonic stem cells.Nat Genet. 2015 May;47(5):469-78. doi: 10.1038/ng.3258. Epub 2015 Mar 30. Nat Genet. 2015. PMID: 25822089 Free PMC article.
-
Advancing physiological maturation in human induced pluripotent stem cell-derived cardiac muscle by gene editing an inducible adult troponin isoform switch.Stem Cells. 2020 Oct 1;38(10):1254-1266. doi: 10.1002/stem.3235. Epub 2020 Jun 16. Stem Cells. 2020. PMID: 32497296 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

