The generation of olfactory epithelial neurospheres in vitro predicts engraftment capacity following transplantation in vivo
- PMID: 21376038
- PMCID: PMC3100381
- DOI: 10.1016/j.expneurol.2011.02.014
The generation of olfactory epithelial neurospheres in vitro predicts engraftment capacity following transplantation in vivo
Abstract
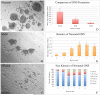
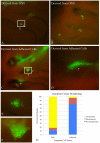
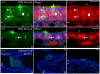
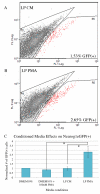
The stem and progenitor cells of the olfactory epithelium maintain the tissue throughout life and effectuate epithelial reconstitution after injury. We have utilized free-floating olfactory neurosphere cultures to study factors influencing proliferation, differentiation, and transplantation potency of sphere-grown cells as a first step toward using them for therapeutic purposes. Olfactory neurospheres form best and expand most when grown from neonatal epithelium, although methyl bromide-injured or normal adult material is weakly spherogenic. The spheres contain the full range of epithelial cell types as marked by cytokeratins, neuron-specific antigens, E-cadherin, Sox2, and Sox9. Globose basal cells are also prominent constituents. Medium conditioned by growth of phorbol ester-stimulated, immortalized lamina propria-derived cells (LP(Imm)) significantly increases the percentage of Neurog1eGFP(+) progenitors and immature neurons in spheres. Sphere-forming capacity resides within selected populations; FACS-purified, Neurog1eGFP(+) cells were poorly spherogenic, while preparations from ΔSox2eGFP transgenic mice that are enriched for Sox2(+) basal cells formed spheres very efficiently. Finally, we compared the potency following transplantation of cells grown in spheres vs. cells derived from adherent cultures. The sphere-derived cells engrafted and produced colonies with multiple cell types that incorporated into and resembled host epithelium; cells from adherent cultures did not. Furthermore, cells from spheres grown in conditioned media from the phorbol ester-activated LP(Imm) line gave rise to significantly more neurons after transplantation as compared with control. The current findings demonstrate that sphere formation serves as a biomarker for engraftment capacity and multipotency of olfactory progenitors, which are requirements for their eventual translational use.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Multipotency of purified, transplanted globose basal cells in olfactory epithelium.J Comp Neurol. 2004 Feb 16;469(4):457-74. doi: 10.1002/cne.11031. J Comp Neurol. 2004. PMID: 14755529
-
Secreted factors from olfactory mucosa cells expanded as free-floating spheres increase neurogenesis in olfactory bulb neurosphere cultures.BMC Neurosci. 2008 Feb 18;9:24. doi: 10.1186/1471-2202-9-24. BMC Neurosci. 2008. PMID: 18282276 Free PMC article.
-
Transcription factor p63 controls the reserve status but not the stemness of horizontal basal cells in the olfactory epithelium.Proc Natl Acad Sci U S A. 2015 Sep 8;112(36):E5068-77. doi: 10.1073/pnas.1512272112. Epub 2015 Aug 25. Proc Natl Acad Sci U S A. 2015. PMID: 26305958 Free PMC article.
-
Identification and molecular regulation of neural stem cells in the olfactory epithelium.Exp Cell Res. 2005 Jun 10;306(2):309-16. doi: 10.1016/j.yexcr.2005.03.027. Epub 2005 Apr 21. Exp Cell Res. 2005. PMID: 15925585 Review.
-
Olfactory epithelium progenitors: insights from transgenic mice and in vitro biology.J Mol Histol. 2007 Dec;38(6):581-99. doi: 10.1007/s10735-007-9141-2. Epub 2007 Sep 13. J Mol Histol. 2007. PMID: 17851769 Review.
Cited by
-
Contribution of Polycomb group proteins to olfactory basal stem cell self-renewal in a novel c-KIT+ culture model and in vivo.Development. 2016 Dec 1;143(23):4394-4404. doi: 10.1242/dev.142653. Epub 2016 Oct 27. Development. 2016. PMID: 27789621 Free PMC article.
-
Spheroid Culture of Mammalian Olfactory Receptor Neurons: Potential Applications for a Bioelectronic Nose.Exp Neurobiol. 2018 Dec;27(6):574-592. doi: 10.5607/en.2018.27.6.574. Epub 2018 Dec 28. Exp Neurobiol. 2018. PMID: 30636907 Free PMC article.
-
Organoid models for chemosensing cell studies.Am J Physiol Cell Physiol. 2025 Jul 1;329(1):C136-C144. doi: 10.1152/ajpcell.00047.2025. Epub 2025 Jun 6. Am J Physiol Cell Physiol. 2025. PMID: 40478437 Free PMC article. Review.
-
Isolation and Differentiation of Neurons and Glial Cells from Olfactory Epithelium in Living Subjects.Mol Neurobiol. 2023 Aug;60(8):4472-4487. doi: 10.1007/s12035-023-03363-2. Epub 2023 Apr 28. Mol Neurobiol. 2023. PMID: 37118325 Free PMC article.
-
Role and Mechanism of Olfactory Stem Cells in the Treatment of Olfactory Disorders.Stem Cells Int. 2025 Apr 24;2025:6631857. doi: 10.1155/sci/6631857. eCollection 2025. Stem Cells Int. 2025. PMID: 40313858 Free PMC article. Review.
References
-
- Barraud P, He X, Zhao C, Ibanez C, Raha-Chowdhury R, Caldwell MA, Franklin RJ. Contrasting effects of basic fibroblast growth factor and epidermal growth factor on mouse neonatal olfactory mucosa cells. Eur J Neurosci. 2007;26:3345–3357. - PubMed
-
- Chen X, Fang H, Schwob JE. Multipotency of purified, transplanted globose basal cells in olfactory epithelium. J Comp Neurol. 2004;469:457–474. - PubMed
-
- Dammann CE, Nielsen HC, Carraway KL., 3rd Role of neuregulin-1 beta in the developing lung. Am J Respir Crit Care Med. 2003;167:1711–1716. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

