Coffee consumption is associated with response to peginterferon and ribavirin therapy in patients with chronic hepatitis C
- PMID: 21376050
- PMCID: PMC3109110
- DOI: 10.1053/j.gastro.2011.02.061
Coffee consumption is associated with response to peginterferon and ribavirin therapy in patients with chronic hepatitis C
Abstract
Background & aims: High-level coffee consumption has been associated with reduced progression of pre-existing liver diseases and lower risk of hepatocellular carcinoma. However, its relationship with therapy for hepatitis C virus infection has not been evaluated.
Methods: Patients (n=885) from the lead-in phase of the Hepatitis C Antiviral Long-Term Treatment Against Cirrhosis Trial recorded coffee intake before retreatment with peginterferon α-2a (180 μg/wk) and ribavirin (1000-1200 mg/day). We assessed patients for early virologic response (2 log10 reduction in level of hepatitis C virus RNA at week 12; n=466), and undetectable hepatitis C virus RNA at weeks 20 (n=320), 48 (end of treatment, n=284), and 72 (sustained virologic response; n=157).
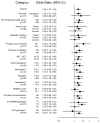
Results: Median log10 drop from baseline to week 20 was 2.0 (interquartile range [IQR], 0.6-3.9) among nondrinkers and 4.0 (IQR, 2.1-4.7) among patients that drank 3 or more cups/day of coffee (P trend<.0001). After adjustment for age, race/ethnicity, sex, alcohol, cirrhosis, ratio of aspartate aminotransferase to alanine aminotransferase, the IL28B polymorphism rs12979860, dose reduction of peginterferon, and other covariates, odds ratios for drinking 3 or more cups/day vs nondrinking were 2.0 (95% confidence interval [CI]: 1.1-3.6; P trend=.004) for early virologic response, 2.1 (95% CI: 1.1-3.9; P trend=.005) for week 20 virologic response, 2.4 (95% CI: 1.3-4.6; P trend=.001) for end of treatment, and 1.8 (95% CI: 0.8-3.9; P trend=.034) for sustained virologic response.
Conclusions: High-level consumption of coffee (more than 3 cups per day) is an independent predictor of improved virologic response to peginterferon plus ribavirin in patients with hepatitis C.
Trial registration: ClinicalTrials.gov NCT00006164.
Copyright © 2011 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Daclatasvir vs telaprevir plus peginterferon alfa/ribavirin for hepatitis C virus genotype 1.World J Gastroenterol. 2016 Mar 28;22(12):3418-31. doi: 10.3748/wjg.v22.i12.3418. World J Gastroenterol. 2016. PMID: 27022224 Free PMC article. Clinical Trial.
-
Effect of HCV RNA suppression during peginterferon alfa-2a maintenance therapy on clinical outcomes in the HALT-C trial.Gastroenterology. 2009 Dec;137(6):1986-94. doi: 10.1053/j.gastro.2009.08.067. Epub 2009 Sep 10. Gastroenterology. 2009. PMID: 19747918 Free PMC article. Clinical Trial.
-
Factors associated with early virological response to peginterferon-α-2a/ribavirin in chronic hepatitis C.World J Gastroenterol. 2013 Mar 28;19(12):1943-52. doi: 10.3748/wjg.v19.i12.1943. World J Gastroenterol. 2013. PMID: 23569340 Free PMC article.
-
Peginterferon alpha and ribavirin combination therapy in patients with hepatitis C virus-related liver cirrhosis.Korean J Hepatol. 2011 Sep;17(3):220-5. doi: 10.3350/kjhep.2011.17.3.220. Korean J Hepatol. 2011. PMID: 22102389 Free PMC article.
-
Durability of a sustained virological response, late clinical sequelae, and long-term changes in aspartate aminotransferase to the platelet ratio index after successful treatment with peginterferon/ribavirin for chronic hepatitis C: a prospective study.Eur J Gastroenterol Hepatol. 2013 Jul;25(7):798-805. doi: 10.1097/MEG.0b013e32835eb8bf. Eur J Gastroenterol Hepatol. 2013. PMID: 23395996
Cited by
-
Regular coffee intake improves liver enzyme levels and liver histology in patients with chronic alcohol consumption, non-alcoholic fatty liver and non-alcoholic steatohepatitis: Report of 259 cases.Hepatol Forum. 2020 Sep 21;1(3):88-96. doi: 10.14744/hf.2020.2020.0026. eCollection 2020 Sep. Hepatol Forum. 2020. PMID: 35949725 Free PMC article.
-
Coffee Intake and Neurocognitive Performance in HIV/HCV Coinfected Patients (ANRS CO13 HEPAVIH).Nutrients. 2020 Aug 21;12(9):2532. doi: 10.3390/nu12092532. Nutrients. 2020. PMID: 32825538 Free PMC article.
-
Coffee intake linked to improved SVR in patients undergoing treatment for hepatitis C.Ann Gastroenterol. 2011;24(4):333-334. Ann Gastroenterol. 2011. PMID: 24713793 Free PMC article. No abstract available.
-
Processes to manage analyses and publications in a phase III multicenter randomized clinical trial.Trials. 2014 May 7;15:159. doi: 10.1186/1745-6215-15-159. Trials. 2014. PMID: 24886378 Free PMC article. Clinical Trial.
-
Individualized therapy for hepatitis C infection: focus on the interleukin-28B polymorphism in directing therapy.Mol Diagn Ther. 2014 Feb;18(1):25-38. doi: 10.1007/s40291-013-0053-4. Mol Diagn Ther. 2014. PMID: 24022240 Review.
References
-
- Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29 (Suppl 1):74–81. - PubMed
-
- Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. - PubMed
-
- Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. - PubMed
-
- Kau A, Vermehren J, Sarrazin C. Treatment predictors of a sustained virologic response in hepatitis B and C. J Hepatol. 2008;49:634–651. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- N01-DK-9-2325/DK/NIDDK NIH HHS/United States
- 1 UL1 RR024986/RR/NCRR NIH HHS/United States
- 1 UL1 RR025758-01/RR/NCRR NIH HHS/United States
- N01-DK-9-2321/DK/NIDDK NIH HHS/United States
- 1 UL1 RR024982-01/RR/NCRR NIH HHS/United States
- N01-DK-9-2320/DK/NIDDK NIH HHS/United States
- M01 RR006192/RR/NCRR NIH HHS/United States
- M01 RR001066/RR/NCRR NIH HHS/United States
- M01 RR000633/RR/NCRR NIH HHS/United States
- M01 RR000065/RR/NCRR NIH HHS/United States
- UL1 RR024982/RR/NCRR NIH HHS/United States
- N01-DK-9-2322/DK/NIDDK NIH HHS/United States
- M01 RR000043/RR/NCRR NIH HHS/United States
- M01 RR000827/RR/NCRR NIH HHS/United States
- UL1 RR024986/RR/NCRR NIH HHS/United States
- M01RR-00042/RR/NCRR NIH HHS/United States
- 1 UL1 RR 025780-01/RR/NCRR NIH HHS/United States
- M01RR-00065/RR/NCRR NIH HHS/United States
- N01-DK-9-2328/DK/NIDDK NIH HHS/United States
- N01-DK-9-2318/DK/NIDDK NIH HHS/United States
- N01-DK-9-2324/DK/NIDDK NIH HHS/United States
- N01-DK-9-2319/DK/NIDDK NIH HHS/United States
- M01RR-00051/RR/NCRR NIH HHS/United States
- UL1 RR025780/RR/NCRR NIH HHS/United States
- M01RR-00827/RR/NCRR NIH HHS/United States
- N01-DK-9-2327/DK/NIDDK NIH HHS/United States
- M01RR-01066/RR/NCRR NIH HHS/United States
- M01 RR000051/RR/NCRR NIH HHS/United States
- N01-DK-9-2326/DK/NIDDK NIH HHS/United States
- Z99 CA999999/ImNIH/Intramural NIH HHS/United States
- N01-DK-9-2323/DK/NIDDK NIH HHS/United States
- UL1 RR025758/RR/NCRR NIH HHS/United States
- M01 RR000042/RR/NCRR NIH HHS/United States
- M01RR-00633/RR/NCRR NIH HHS/United States
- M01RR-00043/RR/NCRR NIH HHS/United States
- M01RR-06192/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

